Packages (Simulation)

Reagent Preparation
Image (I)
Image (II)
Certificate


- Featured-Product
ELISA Kit for Interleukin 8 (IL8)
CXCL8; AMCF-I; GCP1; K60; LECT; LUCT; LYNAP; MDNCF; MONAP; NAF; NAP1; SCYB8; TSG1; B-ENAP; Neutrophil-Activating Protein 1; Granulocyte Chemotactic Protein 1
- Product No.SEA080Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3h
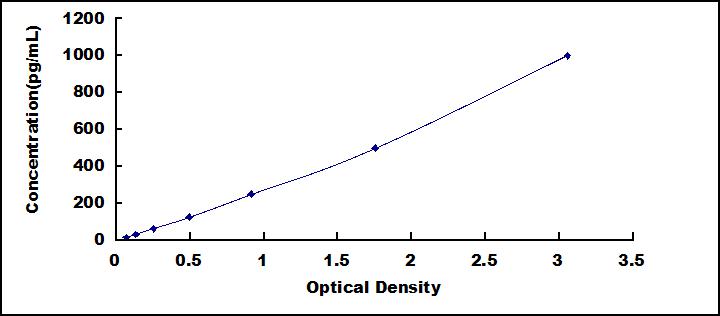
- Detection Range15.6-1,000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 6.0pg/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 349
US$ 498
US$ 2241
US$ 4233
US$ 34860
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Interleukin 8 (IL8).
No significant cross-reactivity or interference between Interleukin 8 (IL8) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Interleukin 8 (IL8) and the recovery rates were calculated by comparing the measured value to the expected amount of Interleukin 8 (IL8) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 88-102 | 96 |
| EDTA plasma(n=5) | 79-91 | 87 |
| heparin plasma(n=5) | 97-104 | 101 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Interleukin 8 (IL8) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Interleukin 8 (IL8) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Interleukin 8 (IL8) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 91-101% | 79-93% | 97-105% | 95-103% |
| EDTA plasma(n=5) | 93-101% | 86-103% | 80-91% | 82-103% |
| heparin plasma(n=5) | 80-101% | 81-93% | 78-93% | 96-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
GIVEAWAYS
INCREMENT SERVICES
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution -
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
| Magazine | Citations |
| Reproductive Biology and Endocrinology | Evidence that polymorphonuclear neutrophils infiltrate into the developing corpus luteum and promote angiogenesis with interleukin-8 in the cow Rbej: 14777827 |
| Acta Otolaryngol | Serial cytokine levels during wound healing in rabbit maxillary sinus mucosa PubMed: 19958244 |
| Journal of Clinical Periodontology | Experimental peri-implant mucositis at different implant surfaces. Pubmed: 24521508 |
| Physiological Research | Budesonide added to modified porcine surfactant Curosurf may additionally improve the lung functions in meconium aspiration syndrome. Pubmed: 2432969 |
| Rheumatology International | Serum levels of selected chemokines in systemic lupus erythematosus patients Pubmed: 22461186 |
| J Med Life. | Expression of interleukine-8 as an independent prognostic factor for sporadic colon cancer dissemination Pubmed:Pmc4197484 |
| J Clin Periodontol. | Experimental peri‐implant mucositis at different implant surfaces Pubmed:24521508 |
| British Journal of Nutrition | Grape seed extract supplementation attenuates the heat stress-induced responses of jejunum epithelial cells in Simmental?×?Qinchuan steers Pubmed:24846452 |
| Theriogenology | Inflammatory cytokine concentrations in uterine flush and serum samples from dairy cows with clinical or subclinical endometritis Theriojournal:Source |
| Anim Reprod Sci. | Alteration in peripheral blood concentration of certain pro-inflammatory cytokines in cows developing retention of fetal membranes PubMed: 25851495 |
| Медицина и здравоохранение | ДИНАМИКА ЦИТОКИНОВОГО ПРОФИЛЯ В ОЦЕНКЕ ЭФФЕКТИВНОСТИ РАЗНЫХ МЕТОДОВ СТИМУЛЯЦИИ РЕПАРАТИВНОГО ОСТЕОГЕНЕЗА В ЭКСПЕРИМЕНТЕ Article: N |
| BMC Anesthesiology | Hyperinflation deteriorates arterial oxygenation and lung injury in a rabbit model of ARDS with repeated open endotracheal suctioning PubMed: 25943099 |
| J Breath Res | Effects of different ventilation strategies on exhaled nitric oxide in geriatric abdominal surgery PubMed: 25719610 |
| ARTICLE | The Predictive Diagnostic and Prognostic Cut-off Values for Interleukin 8 in Patients with Meningitis in Egypt Profile: Mohmad_Mosaad |
| Veterinary Immunology and Immunopathology | Neutrophil gene dynamics and plasma cytokine levels in dairy cattle during peri-implantation period Pubmed:27090626 |
| Infection, Genetics and Evolution | Infection of chicken bone marrow mononuclear cells with subgroup J avian leukosis virus inhibits dendritic cell differentiation and alters cytokine expression Pubmed:27349993 |
| Asian Pacific Journal of Tropical Medicine | Perfusion of gastrodin in abdominal aorta for alleviating spinal cord ischemia reperfusion injury science:S1995764516300955 |
| Archives of Biological Sciences | Diabetes-induced renal failure is associated with tissue inflammation and neutrophil gelatinase-associated lipocalin: Effects of resveratrol publication:299492265_Diabetes-induced_renal_failu |
| Journal of Agricultural Biotechnology | Gln Attenuates LPS-induced Inflammatory Injury of Bos taurus Testis Sustentacular Cells 10610 |
| journal of molecular and cellular cardiology | Mutant DD genotype of NFKB1 gene is associated with the susceptibility and severity of coronary artery disease. pubmed:28088561 |
| Physiological Research | Intravenous dexamethasone attenuated inflammation and influenced apoptosis of lung cells in an experimental model of acute lung injury. pubmed:28006948 |
| PLOS ONE | Mechanical Ventilation Alters the Development of Staphylococcus aureus Pneumonia in Rabbit. pubmed:27391952 |
| The Journal of Comenius University in Bratislava | Effects of Conventional Mechanical Ventilation Performed by Two Neonatal Ventilators on the Lung Functions of Rabbits with Meconium-Induced Acute Lung Injury acm.2017.16.issue-3 |
| Journal of Physiology and Pharmacology | Effects of budesonide on the lung functions, inflammation and apoptosis in a saline-lavagemodel of acute lung injury. pubmed:28195073 |
| 中国药物警戒 | 小剂量阿奇霉素对稳定期老年慢性阻塞性肺疾病患者外周血内TNF-α、IL-8、CRP水平及肺功能的影响 Periodical_zgywjj201608004 |
| Veterinary Immunology and Immunopathology | Cytokine levels in colostrum and in foals' serum pre- and post-suckling pubmed:28242000 |
| Journal of Cellular Physiology | Increased Chondrogenic Potential of Mesenchymal Cells From Adipose Tissue Versus Bone Marrow‐Derived Cells in Osteoarthritic In Vitro Models pubmed:27739057 |
| European Journal of Pharmacology | Cardioprotective time-window of Penehyclidine hydrochloride postconditioning: A rat study pubmed:28684235 |
| PLoS One | Linezolid and atorvastatin impact on pneumonia caused by Staphyloccocus aureus in rabbits with or without mechanical ventilation pubmed:29149185 |
| Archives of Oral Biology | The effect of restorative materials on cytokines in gingival crevicular fluid pubmed:28992599 |
| Experimentelle periimplantäre Mukositis an unterschiedlichen Implantatoberflächen doi: 10.1111/jcpe.12240. | |
| Molecular Medicine Reports | CagA promotes proliferation and inhibits apoptosis of GES-1 cells by upregulating TRAF1/4-1BB pubmed:28627614 |
| ARTIFICIAL CELLS NANOMEDICINE AND BIOTECHNOLOGY | Dexamethasone reduces serum level of IL-17 in Bleomycin-A5-induced rats model of pulmonary fibrosis pubmed:28608724 |
| Biological Rhythm Research | Alteration in some pro and anti-inflammatory cytokines associated with complete and incomplete gestation cycle of cows 10.1080/09291016.2017.1319636 |
| Scientific Reports | Optimization of Storage Temperature for Retention of Undifferentiated Cell Character of Cultured Human Epidermal Cell Sheets pubmed:28811665 |
| Journal of Ultrasound in Medicine | Inhibitory Effect of Low-Intensity Pulsed Ultrasound on the Expression of Lipopolysaccharide-Induced Inflammatory Factors in U937 Cells. pubmed:28600899 |
| Veterinary Immunology and Immunopathology | Integrated effect of seasons and lactation stages on the plasma inflammatory cytokines, function and receptor expression of milk neutrophils in Sahiwal (Bos indicus) cows. pubmed:28895861 |
| Biomedicine hub | Comparison of Polymyxin E and Polymyxin B as an Additive to Pulmonary Surfactant in Escherichia coli Pneumonia of Ventilated Neonatal Rabbits 10.1159/000475877 |
| Anticancer Research | Association Between Endothelial Progenitor Cells and Treatment Response in Non-Squamous Non-small Cell Lung Cancer Treated with Bevacizumab. pubmed:28982871 |
| Chronic Respiratory Disease | Inflammatory biomarkers and radiologic measurements in never-smokers with COPD: A cross-sectional study from the CODA cohort. pubmed:29117798 |
| Inflammopharmacology | Polar extract of protects cartilage homeostasis: possible mechanism of action Pubmed:29313174 |
| Life Sciences | Dexmedetomidine attenuates renal fibrosis via α2-adrenergic receptor-dependent inhibition of cellular senescence after renal ischemia/reperfusion Pubmed:29729264 |
| Microbial Pathogenesis | Pathogen-dependent modulation of milk neutrophils competence, plasma inflammatory cytokines and milk quality during intramammary infection of Sahiwal (Bos … Pubmed:29787791 |
| Lasers in Medical Science | Evaluation of the therapeutic effect of micro-plasma radio frequency on hypertrophic scars in rabbit ears Pubmed:30003425 |
| Medical Science Monitor | Rho Kinase Type 1 (ROCK1) Promotes Lipopolysaccharide-induced Inflammation in Corneal Epithelial Cells by Activating Toll-Like Receptor 4 (TLR4) … Pubmed:29804125 |
| Journal of Veterinary Science | Rifaximin anti‐inflammatory activity on bovine endometrium primary cell cultures: a preliminary study Pubmed:29984902 |
| Bioscience Reports | N-Acetylcysteine inhalation improves pulmonary function in patients received Liver Transplantation. Doi: 10.1042/BSR20180858 |
| Acta Medica Mediterranea | Effects of Conventional Mechanical Ventilation Performed by Two Neonatal Ventilators on the Lung Functions of Rabbits with Meconium-Induced Acute Lung … Doi: 10.1515/acm-2016-0012 |
| Journal of Dairy Science | Metabolism and immune status during transition period influences the lactation performance in Zebu (Bos indicus () cows Pubmed: 17430930 |
| Biomedicine & Pharmacotherapy | Interleukin 8 targeted contrast echocardiography is effective to evaluate myocardial ischemia-reperfusion injury in the rabbits |
| Emerging Microbes & Infections | Immunogenicity and protective efficacy against Treponema pallidum in New Zealand rabbits immunized with plasmid DNA encoding flagellin Pubmed: 30405111 |
| Journal of Investigative Medicine | The characteristics and clinical significance of mucin levels in bronchoalveolar lavage fluid of patients with interstitial lung disease Pubmed: 30573494 |
| International Journal of Chronic Obstructive Pulmonary Disease | Altered serum levels of type I collagen turnover indicators accompanied by IL-6 and IL-8 release in stable COPD Pubmed: 30655663 |
| Inflammopharmacology | Chandrasekaran Chinampudur Velusami, Edwin Jothie Richard & |
| Journal of Hematology & Oncology | Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment … Pubmed: 30621731 |
| BioMed Research International | Effective Treatment of Bovine Mastitis with Intramammary Infusion of Angelica dahurica and Rheum officinale Extracts |
| Frontiers in Pharmacology | Naoxintong Capsule Inhibits the Development of Cardiovascular Pathological Changes in Bama Minipig Through Improving Gut Microbiota Pubmed: 31632272 |
| international journal of molecular sciences | Anti-Inflammatory and Anti-Apoptotic Effects of Stybenpropol A on Human Umbilical Vein Endothelial Cells Pubmed: 31671764 |
| Scientific Reports | Corrosion Resistance of Graphene oxide/Silver Coatings on Ni–Ti alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts Pubmed: 32094428 |
| Journal of Receptors and Signal Transduction Research | Suppressor of ras val-2 promotes inflammation-mediated oxidative stress and cell apoptosis in cardiomyocytes through activating Mst1-mROS signaling pathway Pubmed: 32065019 |
| Antibiotics | Effects of Early Intervention with Antibiotics and Maternal Fecal Microbiota on Transcriptomic Profiling Ileal Mucusa in Neonatal Pigs Pubmed: 31963653 |
| Physiol Res | Effect of Different Dosages of Dexamethasone Therapy on Lung Function and Inflammation in an Early Phase of Acute Respiratory Distress Syndrome Model Pubmed: 31928043 |
| Respiratory Research | Synthetic surfactant with a recombinant surfactant protein C analogue improves lung function and attenuates inflammation in a model of acute respiratory … Pubmed: 31694668 |
| Journal of Pharmacy and Pharmacology | Tectorigenin inhibits inflammation and pulmonary fibrosis in allergic asthma model of ovalbumin‐sensitized guinea pigs Pubmed: 32314371 |
| Inflammation | Cyanidin-3-O-Glucoside Attenuates Lipopolysaccharide-Induced Inflammation in Human Corneal Epithelial Cells by Inducing Let-7b-5p-Mediated HMGA2/PI3K/Akt … Pubmed: 32248330 |
| Sci Rep | NFKB1 gene rs28362491 ins/del variation is associated with higher susceptibility to myocardial infarction in a Chinese Han population Pubmed: 33177541 |
| International Journal of Nanomedicine | Study on Adenovirus Infection in vitro with Nanoself-Assembling Peptide as Scaffolds for 3D Culture Pubmed: 32922004 |
| Theriogenology | A comparative study on various immunological parameters influencing embryo survivability in crossbred dairy cows Pubmed: 32810791 |
| Cell Cycle | SIRT1 relieves Necrotizing Enterocolitis through inactivation of Hypoxia-inducible factor (HIF)-1a Pubmed: 32657204 |
| Frontiers in Pharmacology | Hyperoside Protects Human Umbilical Vein Endothelial Cells Against Anticardiolipin Antibody-Induced Injury by Activating Autophagy Pubmed: 32508661 |
| Indian Journal of Animal Sciences | Peripheral concentrations of metabolic and inflammatory indicators during transition period and their relationship with postpartum clinical endometritis in dairy … |
| Animals | Diet Supplementation with a Bioactive Pomace Extract from Olea europaea Partially Mitigates Negative Effects on Gut Health Arising from a Short-Term Fasting Period … |
| INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE | Possible Anti-inflammatory Effects of Mesenchymal Stem Cells Transplantation via Changes in CXCL8 Levels in Patients with Refractory Rheumatoid Arthritis |
| HMGB1 e citocinas inflamatórias em lesão pulmonar aguda experimental induzida em coelhos | |
| Bull Exp Biol Med | Inflammation Mediators Regulate the Microbiota Resistance to Adverse Factors 33222085 |
| physiological reports | Impact of synthetic surfactant CHF5633 with SP©\B and SP©\C analogues on lung function and inflammation in rabbit model of acute respiratory distress syndrome 33403805 |
| J Med Food | Cough Inhibition Activity of Schisandra chinensis in Guinea Pigs 33861937 |
| Sci Rep | Association between MIF gene promoter rs755622 and susceptibility to coronary artery disease and inflammatory cytokines in the Chinese Han population 33850223 |
| Sci Rep | Corrosion Resistance of Graphene oxide/Silver Coatings on Ni¨CTi alloy and Expression of IL-6 and IL-8 in Human O... 32094428 |
| Poultry Science | Response of broiler chickens fed diets supplemented with a bioactive olive pomace extract from Olea europaea to an experimental coccidial vaccine?¡ 33518110 |
| Bioactive Materials | Construction of heparin-based hydrogel incorporated with Cu5. 4O ultrasmall nanozymes for wound healing and inflammation inhibition 33778192 |
| animals | Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic?¡ 34066204 |
| Sci Rep | Talaromyces marneffei and nontuberculous mycobacteria co-infection in HIV-negative patients 34376749 |
| Pharmaceutics | Nitric-Oxide-Releasing Dexamethasone Derivative NCX-1005 Improves Lung Function and Attenuates Inflammation in Experimental Lavage-Induced ARDS 34959373 |
| Biomedicines | Exploring Epithelial–Mesenchymal Transition Signals in Endometriosis Diagnosis and In Vitro Fertilization Outcomes 34829910 |
| Anesthesiology | Adverse Mechanical Ventilation and Pneumococcal Pneumonia Induce Immune and Mitochondrial Dysfunctions Mitigated by Mesenchymal Stem Cells in Rabbits Pubmed:34965287 |
| J Clin Med | Endometrial Dysbiosis Is Related to Inflammatory Factors in Women with Repeated Implantation Failure: A Pilot Study Pubmed:35566605 |
| Microb Pathog | Cell damage and neutrophils promote the infection of Mycoplasma pneumoniae and inflammatory response Pubmed:35724831 |
| ResearchSquare | LncRNA KCNQ10T1 shuttled by bone marrow mesenchymal stem cells-derived exosome inhibits sepsis via regulation of miR-154-3p/RNF19A axis |
| Anti-inflammatory activity of non-selective PDE inhibitor aminophylline on the lung tissue and respiratory parameters in animal model of ARDS | |
| Intensive Care Research | Evaluating the Roles of sCD14 and sCD14-ST in Diagnosing COPD and Predicting an Acute Exacerbation of COPD |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| APA080Hu01 | Active Interleukin 8 (IL8) | Cell culture; Activity Assays. |
| RPA080Hu01 | Recombinant Interleukin 8 (IL8) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAA080Hu01 | Polyclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IP. |
| LAA080Hu71 | Biotin-Linked Polyclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC. |
| LAA080Hu81 | FITC-Linked Polyclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IF. |
| MAA080Hu24 | Monoclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IP. |
| MAA080Hu21 | Monoclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IP. |
| MAA080Hu22 | Monoclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IP. |
| LAA080Hu82 | FITC-Linked Monoclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC; IF. |
| LAA080Hu72 | Biotin-Linked Monoclonal Antibody to Interleukin 8 (IL8) | WB; IHC; ICC. |
| MEA080Hu | Mini Samples ELISA Kit for Interleukin 8 (IL8) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SEA080Hu | ELISA Kit for Interleukin 8 (IL8) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| HEA080Hu | High Sensitive ELISA Kit for Interleukin 8 (IL8) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| IEA080Hu | Instant ELISA Kit for Interleukin 8 (IL8) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| AEA080Hu | ELISA Kit for Anti-Interleukin 8 Antibody (Anti-IL8) | Enzyme-linked immunosorbent assay for Antibody Detection. |
| SCA080Hu | CLIA Kit for Interleukin 8 (IL8) | Chemiluminescent immunoassay for Antigen Detection. |
| LMA080Hu | Multiplex Assay Kit for Interleukin 8 (IL8) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |
| PSA080Hu01 | Antibody Pair for Interleukin 8 (IL8) | ELISA; CLIA; ELISPOT; Luminex; Immunochromatography and other Immunoassays. |
| KSA080Hu01 | ELISA Kit DIY Materials for Interleukin 8 (IL8) | Main materials for "Do It (ELISA Kit) Yourself". |