Experimental Operation Guideline-Trouble Shooting of Western Blot(WB)
Western blot (Western Blot) is a rapid and sensitive technique for detecting the protein of interest in the samples according to the antigen-antibody reactions. Western blot protocol uses gel electrophoresis (SDS-PAGE or native PAGE) to separate proteins in samples based on the difference of molecular weight of proteins . Then the proteins are transferred from the gel onto a membrane (typically nitrocellulose or PVDF), which is non-covalently bound to the proteins and can maintain the biological activity of proteins. After that, the membrane is probed using a primary antibody to detect the protein of interest followed by a further incubation with a secondary antibody conjugated to a reporter molecule. At the end of the assay, the reporter molecules will allow the visualization of the target protein. This technology is widely used to make the semi-quantitative analysis of target protein in biological fluids.
There are three steps in Western Blot, including electrophoresis to separate proteins, Tranmembrane and visualization protein in gels. Each steps may influence the results. To achieve the desired results, every step should be taken great care. The common problems in Western Blot are listed below. In order to improve the results, analysis and suggestions for the problems are shown below.
1. Problems in electrophoresis.
The first step of Western blot is SDS-PAGE. Some problems in Western blot may caused by the operation in electrophoresis. The problems in electrophoresis are listed below.
Table1. The problems and corresponding suggestions in electrophoresis
| Problems | Reasons | Suggestions | Image |
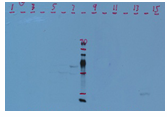
Smile effect on the band( ) ) |
The concentration of gel may be too high;
Uneven surface of resolving gel or the pore size are inconsistent throughout the gel;
Temperature of migration process is too high.
|
Change concentration of gel , Use a gel with higher concentration for small proteins and a lower concentration for large proteins;
Confirm the gel is completely polymerized;
Run the gel in the water bath incubator or on ice.
|
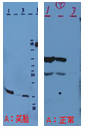 |
Frown effect on band( ) ) |
Air bubble trapped in membrane. | Remove the bubbles when preparing the gel for transmembrane process. | 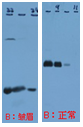 |
| Trailing | Presence of precipitate/ aggragate in samples prior to electrophoresis;
Gel percentage is too high.
|
Centrifuge or filter the sample to remove precipitate before loading;
Use alternative buffer system, add chaotropic agent in samples;
Use fresh buffers;
Decrease the gel concentration.
|
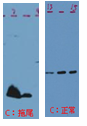 |
| Curved effect on the bands | Presence of precipitate/ aggragate in samples prior to electrophoresis. | Centrifuge or filter the sample to remove particles before loading;
Add chaotropic agent in samples.
|
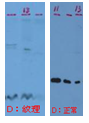 |
| Skewed or distorted bands | The electrophoresis device is skew;
The gel surface around sample wells is uneven.
|
Make sure the electrode is in the right orientation;
Take off the combs slightly to ensure the sample wells are even.
|
|
| Diffuse Bands | Sample overloaded. | Reduce amount of protein loaded. | 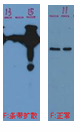 |
2. Problems in Transfer of proteins.
Transmembrane process is the key point for Western blot. Poor or over transfer will lead to the weak signal or no signal. Optimize the transmembrane system can improve the results.
Table2. The problems and corresponding suggestions in transfer of protein
| Problems | Suggestions |
| Poor or incomplete transfer |
Ensure transfer is complete, stain the membrane with Ponceau S;
Optimize transfer time and electric current, High MW protein may require more time for transfer;
Add positive control and/or Marker;
Wet the membrane in transfer buffer according to the manual before the transmembrane process;
Ensure that there is good contact between the membrane and gel.
|
| Over transfer | Reduce time of transfer, especially for low molecular weight proteins (<10kKDa). |
3. Problems in visualization protein in gels.
There are some problems in visualization protein in gels. Below is the analysis and suggestions for the problems.
Table3. The problems and corresponding suggestions in visualization protein in gels
| Problems | Reasons | Suggestion |
|
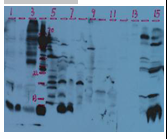
Faint Bands (Weak Signal) or No Bands Observed;
|
Poor transfer of protein to membrane; | Shown in table 2. |
| Insufficient protein; |
Increase the amount of total protein loaded on gel;
Use a positive control;
Switch blocking reagents to avoid the antigen be masked by the blocking buffer.
|
|
| Insufficient antibody; |
ncrease antibody concentration since the antibody may have low affinity to protein of interest;
Antibody may have lost activity, perform a test to determine activity or test alternate antibodies.
|
|
| Insufficient incubation time; | Extent incubation time to overnight at 4°C. | |
| Mismatched primary and secondary antibodies; |
Replace primary/secondary antibodies pairs;
The host of primary antibody needs to be taken into account when selecting the secondary antibodies.
|
|
| Excessive antibodies; | Excessive antibodies can cause extremely high levels of localized signal, which results in rapid, complete consumption of substance at a point. | |
| Sodium Azide contamination; |
Sodium azide inactivates horseradish peroxidase (HRP);
Make sure buffers do not contain Sodium Azide.
|
|
| Excessive washing of the membrane; |
Do not over-wash the membrane;
Add a detergent such as Tween20 to the incubation and washing buffer;
Use detergents with proper concentration.
|
|
| Insufficient exposure time; | Extent exposure time. | |
| Insufficient substrate incubation time; | Extent substrate incubation time. | |
| Inactive substrate. |
Perform a test to determine activity of substrate, switch the substrate if it have lost the activity;
Make sure there is no contamination between the two substrate reagents.
|
|
|
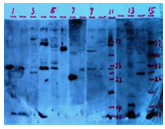
High Background;
|
Insufficient blocking; |
Extent blocking time;
Select the proper blocking reagents.
|
| Improper dilution rate of primary antibody; | Dilute he primary antibodies to determine the proper dilution rate. | |
| The incubation temperature may be too high; | Extent incubation time to overnight at 4°C. | |
| Your choice of membrane; | Select the proper membrane, nitrocellulose membranes are considered to give less background than PVDF. | |
| Membrane dried in the blotting procedure; | Make sure membranes are thoroughly wet through all the processes.. | |
|
Uneven staining of the gel
|
Insufficient blocking of nonspecific sites; |
Optimize the blocking buffer system;
Increase the concentration of blocking reagent;
Optimize the blocking time and temperature;
Add 0.05% Tween-20 to blocking buffer.
|
| Cross-reaction between the blocking agent and the primary or secondary antibodies; |
Switch blocking reagent;
Do not use milk to block membranes when using an avidin-biotin system — milk contains biotin;
Arrange a cross-reaction assay.
|
|
| Antibodies concentration are too high; | Dilute the primary and/or secondary antibodies. | |
| Insufficient washing; |
Reduce the HRP level;
Add 0.05% Tween-20 to blocking buffer;
Increase wash times and the volume of buffer used.
|
|
| Wrong Operation habits; |
Make sure membranes are wet thoroughly;
Ensure uniform agitation by placing on a rocker/shaker;
Care should be taken to prevent the membrane damage when clamping the membrane;
Do not handle membrane with bare hands, always wear clean gloves or use forceps.
|
|
| Film overexposed. | Reduce the exposure time. | |
| Contamination of buffers; |
Use freshly prepared buffers;
Filter buffers before use.
|
|
| Contaminated equipment. |
Make sure electrophoresis equipment, blotting equipment and incubation trays are clean;
Protein or pieces of gel remaining on the unit may stick to the membrane;
Wash membrane thoroughly.
|
|
|
Extra Bands
|
The protein of interest has multiple modified forms in vivo such as acetylation, methylation, myristylation, phosphorylation, glycosylation etc.; | Examine the literature and make bioinformatic analysis of Amino acid modification to determine the apparent size of analytes. |
| Different splice variants that share similar epitopes; | Check the literature and make bioinformatic analysis. | |
| Degradation of Analyte; |
Add protease inhibitors to sample before storage;
Make sample treatment on ice.
|
|
| Primary antibody quality; |
Try different antibodies or select high-specific antibodies;
Purify the antibodies.
|
|
| Primary or secondary antibodies concentration are two high. | Reduce antibody concentration. |
For more information, please visit http://www.cloud-clone.us/.