New findings on the mechanism of melanoma
Melanoma is the most lethal type of skin cancer that originates from the malignant transformation of melanocytes. Actually, melanocytes are of neuroectodermal origin and then migrate extensively to reside throughout the body, including skin, uveal, mucosa, inner ear, and rectum, displaying as highly dendritic cells to manufacture melanin to defend against photodamage. Although the mortality rate of melanoma patients is prominently reduced in the past few decades due to early diagnosis, proper screening approaches, improved surgery principle and revolutionary advances of targeted therapy and immunotherapy, the prognosis of patients, in particular those with distant metastasis, remains unoptimistic. Therefore, exploring the mechanism of melanoma occurrence and development to develop new treatment methods is of great significance to improve the prognosis of patients.
1. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis
Melanoma brain metastases (MBM) frequently occur in patients with advanced melanoma, but little is known about why melanoma spreads to the brain. Benjamin Izar, Columbia University Irving Medical Center, USA, amd his team performed single-cell/nucleus RNA-seq in 22 treatment-naive MBMs and 10 extracranial melanoma metastases (ECMs) and matched spatial single-cell transcriptomics and T cell receptor (TCR)-seq[1]. Cancer cells from MBMs were more chromosomally unstable, adopted a neuronal-like cell state, and enriched for spatially variably expressed metabolic pathways(Fig.1). Integrated spatial analyses revealed distinct geography of putative cancer immune evasion and evidence for more abundant intra-tumoral B to plasma cell differentiation in lymphoid aggregates in MBM. MBM harbored larger fractions of monocyte-derived macrophages and dysfunctional TOX+CD8+ T cells with distinct expression of immune checkpoints. This study offers important insights into salient MBM biology, identifies putative targets for therapeutic modulation, and a rich reference for the brain metastasis research community.
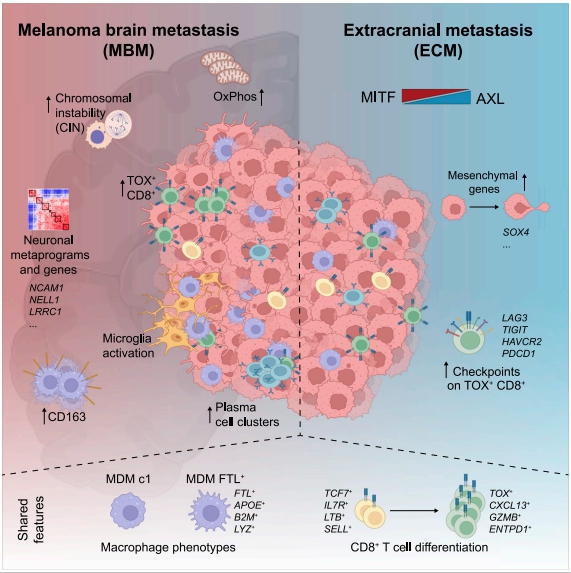
Fig.1 Multi-modal single-cell analysis reveals genomic, transcriptional, and tumor-microenvironmental features of treatment-naive human MBM
2. Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance
MAPK inhibitor (MAPKi) therapy in melanoma leads to the accumulation of tumorsurface PD-L1/ L2, which may evade antitumor immunity and accelerate acquired resistance. Roger S. Lo, Division of Dermatology, Department of Medicine, David Geffen School of Medicine, University of California, California, and his team discover that the E3 ligase ITCH binds, ubiquitinates, and downregulates tumorsurface PD-L1/L2 in MAPKi-treated human melanoma cells, thereby promoting T-cell activation[2]. During MAPKi therapy in vivo, melanoma cell–intrinsic ITCH knockdown induced tumor-surface PD-L1, reduced intratumoral cytolytic CD8+ T cells, and accelerated acquired resistance only in immune-competent mice. Conversely, tumor cell-intrinsic ITCH overexpression reduced MAPKi-elicited PD-L1 accumulation, augmented intratumoral cytolytic CD8+ T cells, and suppressed acquired resistance in melanoma. CD8+ T-cell depletion and tumor cell-intrinsic PD-L1 overexpression nullified the phenotype of ITCH overexpression, thereby supporting an in vivo ITCH-PD-L1-T-cell regulatory axis. The discovery could lead to the development of new treatments for melanoma.
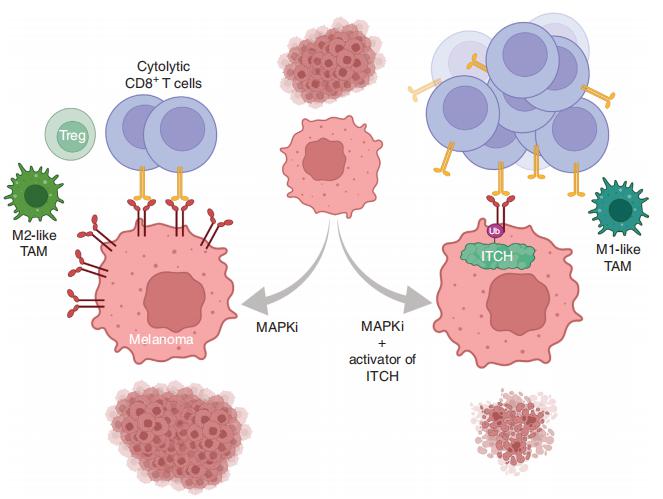
Fig.2 Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance
3. Stromal changes in the aged lung induce an emergence from melanoma dormancy
Disseminated cancer cells from primary tumours can seed in distal tissues, but may take several years to form overt metastases, a phenomenon that is termed tumour dormancy. Ashani T. Weeraratna, Department of Biochemistry and Molecular Biology, Johns Hopkins Bloomberg School of Public Health, USA, and his team showed that the aged lung microenvironment facilitates a permissive niche for efficient outgrowth of dormant disseminated cancer cells—in contrast to the aged skin, in which age-related changes suppress melanoma growth but drive dissemination[3]. They identified WNT5A as an activator of dormancy in melanoma disseminated cancer cells within the lung, which initially enables the efficient dissemination and seeding of melanoma cells in metastatic niches. Age-induced reprogramming of lung fibroblasts increases their secretion of the soluble WNT antagonist sFRP1, which inhibits WNT5A in melanoma cells and thereby enables efficient metastatic outgrowth(Fig.3). They also identified the tyrosine kinase receptors AXL and MER as promoting a dormancy-to-reactivation axis within melanoma cells. These data strongly suggest that we need to consider age as a parameter in the design and delivery of cancer therapy, and as a critical modulator of tumour dormancy and progression.
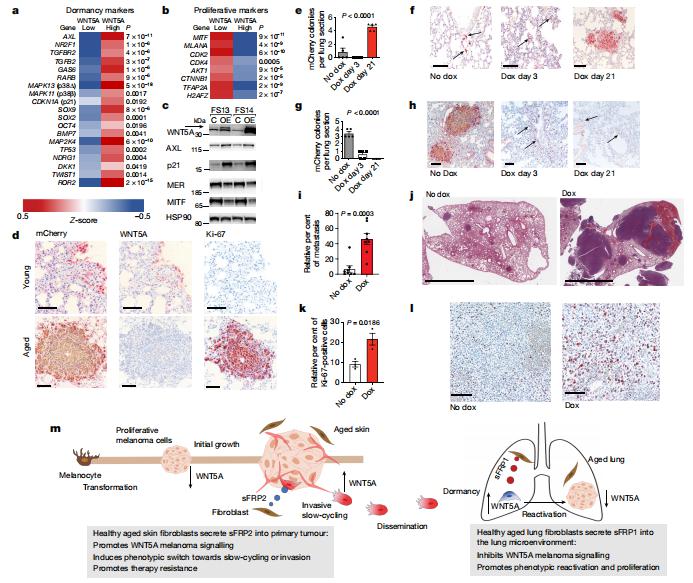
Fig.3 Temporal downregulation of WNT5A promotes metastatic melanoma outgrowth in previously dormant lung microenvironments
4. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy
Treatment with therapy targeting BRAF and MEK (BRAF/MEK) has revolutionized care in melanoma and other cancers; however, therapeutic resistance is common and innovative treatment strategies are needed. Jennifer A. Wargo, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, USA, and his team studied a group of patients with melanoma who were treated with neoadjuvant BRAF/MEK-targeted therapy and observed significantly higher rates of major pathological response and improved recurrence-free survival (RFS) in female versus male patients[4]. The findings were validated in several additional cohorts of patients with unresectable metastatic melanoma who were treated with BRAF- and/or MEK-targeted therapy, demonstrating improved progression-free survival and overall survival in female versus male patients in several of these studies. Studies in preclinical models demonstrated significantly impaired anti-tumour activity in male versus female mice after BRAF/MEK-targeted therapy, with significantly higher expression of the androgen receptor in tumours of male and female BRAF/MEK-treated mice versus the control. Pharmacological inhibition of androgen receptor signalling improved responses to BRAF/MEK-targeted therapy in male and female mice, whereas induction of androgen receptor signalling (through testosterone administration) was associated with a significantly impaired response to BRAF/MEK-targeted therapy in male and female patients(Fig.4). Together, these results have important implications for therapy.
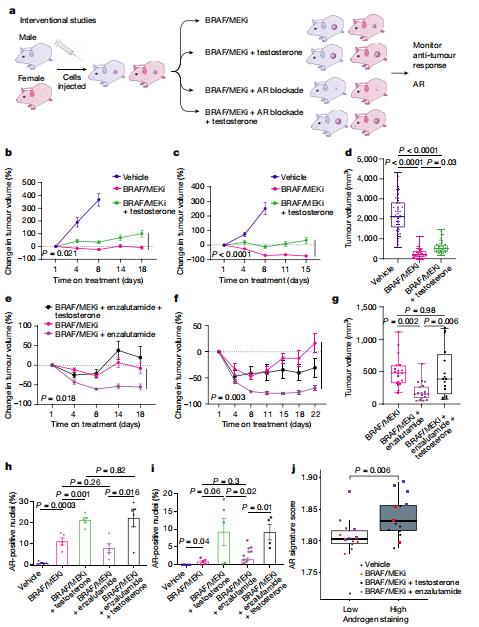
Fig.4 Modulation of AR activity is associated with differential response to BRAF/MEK-targeted therapy
References
[1]Biermann J, Melms JC, Amin AD, et al. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis. Cell. 2022;185(14):2591-2608.e30.(IF=66.850)
[2]Yang Z, Wang Y, Liu S, et al. Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance. Cancer Discov. 2022;12(8):1942-1959.(IF=38.272)
[3]Fane ME, Chhabra Y, Alicea GM, et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature. 2022;606(7913):396-405. (IF=69.504)
[4]Vellano CP, White MG, Andrews MC, et al. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy. Nature. 2022;606(7915):797-803.(IF=69.504)
Cloud clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators and the above-mentioned MAPK, PD-L1, WNT, BRAF and other related products, which can help scientific researchers to conduct cancer-related research.