The role of circadian rhythms in metabolism-related diseases
Circadian rhythm refers to the changes in life activities in a 24-hour cycle. In mammals, the circadian system is organized as a hierarchical oscillator network with the master pacemaker being localized in the suprachiasmatic nucleus (SCN) of the hypothalamus, which coordinates the network of peripheral tissue oscillators. The deterioration of the circadian rhythm can be the main cause of many diseases such as premature mortality, obesity, impaired glucose tolerance, diabetes, psychiatric disorders, anxiety, depression, and cancer progression, fatigue, and loss of concentration. Recently, a number of literatures have reported the mechanisms of circadian rhythm regulating metabolic diseases, providing help for the treatment of corresponding diseases.
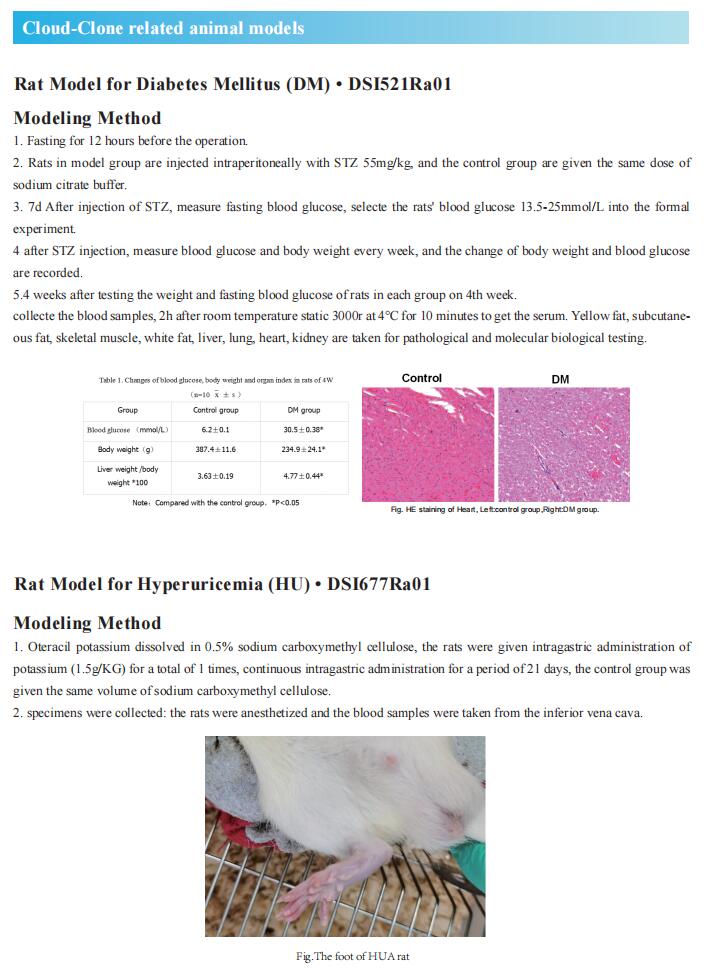
1. Bmal1 regulates production of larger lipoproteins by modulating cAMP-responsive element-binding protein H and apolipoprotein AIV
High plasma lipid/lipoprotein levels are risk factors for various metabolic diseases. Bmal1 is considered to be an important component of the oscillatory network of the circadian system. Xiaoyue Pan and M. Mahmood Hussain, Department of Foundations of Medicine, NYU Long Island School of Medicine, showed that global and liver-specific Bmal1-deficient mice maintained on a chow or Western diet developed hyperlipidemia, denoted by the presence of higher amounts of triglyceride-rich and apolipoprotein AIV (ApoAIV)-rich larger chylomicron and VLDL due to overproduction[1]. Bmal1 deficiency decreased small heterodimer partner (Shp) and increased microsomal triglyceride transfer protein (MTP), a key protein that facilitates primordial lipoprotein assembly and secretion. Moreover, they show that Bmal1 regulates cAMP-responsive element-binding protein H (Crebh) to modulate ApoAIV expression and the assembly of larger lipoproteins. Mechanistic studies showed that Bmal1 regulates Crebh expression by two mechanisms. First, Bmal1 interacts with the Crebh promoter to control circadian regulation. Second, Bmal1 increases Rev-erbα expression, and nuclear receptor subfamily 1 group D member 1 (Nr1D1, Rev-erbα) interacts with the Crebh promoter to repress expression (Fig.1). These results suggest that disruptions in circadian mechanisms contribute to hyperlipidemia and that avoiding disruptions in circadian rhythms may limit/prevent hyperlipidemia and atherosclerosis.
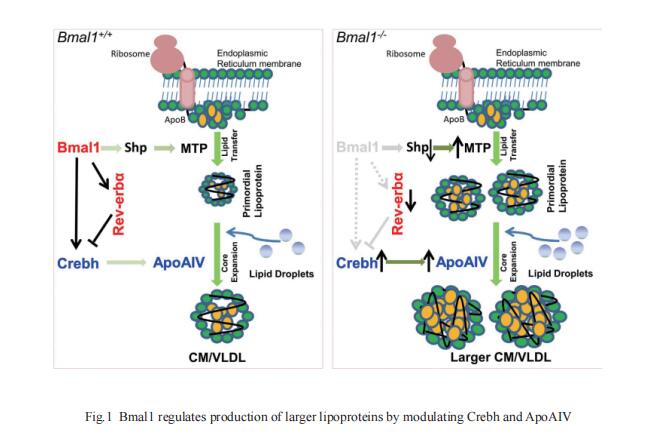
2. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle
Circadian rhythm disruption contributes to type 2 diabetes (T2D) pathogenesis. Juleen R. Zierath,Department of Physiology and Pharmacology, Integrative Physiology, Karolinska Institutet, Sweden, and his team elucidated whether altered circadian rhythmicity of clock genes is associated with metabolic dysfunction in T2D[2]. Transcriptional cycling of circadian rhythm genes BMAL1, CLOCK, and PER3 was altered in skeletal muscle from individuals with T2D, and this was coupled with reduced number and amplitude of cycling genes and disturbed circadian oxygen consumption. Inner mitochondria–associated genes were enriched for rhythmic peaks in normal glucose tolerance, but not T2D, and positively correlated with insulin sensitivity. Chromatin immunoprecipitation sequencing identified CLOCK and BMAL1 binding to inner-mitochondrial genes associated with insulin sensitivity, implicating regulation by the circadian rhythm (Fig.2). Inner-mitochondria disruption altered core-clock gene expression and free-radical production, phenomena that were restored by resveratrol treatment. They identify bidirectional communication between mitochondrial function and rhythmic gene expression, processes that are disturbed in diabetes.
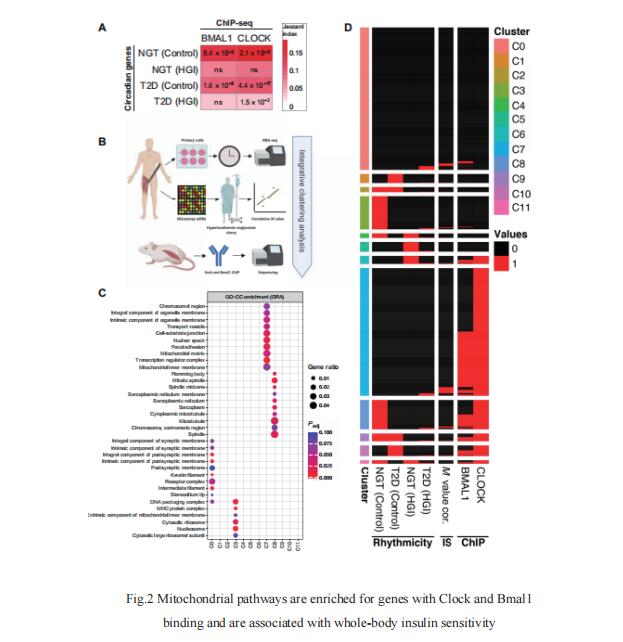
3. REV-ERB nuclear receptors in the SCN control circadian period and restrict diet-induced obesity
REV-ERB nuclear receptors are key components of the molecular clock. Mitchell A. Lazar, Institute for Diabetes, Obesity, and Metabolism and Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Pennsylvania Perelman School of Medicine, USA, and his team reported that mice lacking circadian REV-ERB nuclear receptors in the SCN maintain free-running locomotor and metabolic rhythms, but these rhythms are notably shortened by 3 hours (Fig.3)[3]. When housed under a 24-hour light:dark cycle and fed an obesogenic diet, these mice gained excess weight and accrued more liver fat than controls. These metabolic disturbances were corrected by matching environmental lighting to the shortened endogenous 21-hour clock period, which decreased food consumption. Thus, SCN REV-ERBs are not required for rhythmicity but determine the freerunning period length. More broadly, REV-ERB dysfunction could underlie human circadian desynchrony syndromes, which may be amenable to chronotherapeutic strategies to treat associated metabolic and behavioral disorders.
References
[1]Pan X, Hussain MM. Bmal1 regulates production of larger lipoproteins by modulating cAMP-responsive element-binding protein H and apolipoprotein AIV [J]. Hepatology. 2021;10.1002/hep.32196. (IF=17.425)
[2]Gabriel BM, Altıntaş A, Smith JAB, et al. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle [J]. Sci Adv. 2021;7(43):eabi9654. (IF=14.136)
[3]Adlanmerini M, Krusen BM, Nguyen HCB, et al. REV-ERB nuclear receptors in the suprachiasmatic nucleus control circadian period and restrict diet-induced obesity [J]. Sci Adv. 2021;7(44):eabh2007. (IF=14.136)
Cloud-Clone can not only provide animal models of various metabolic disorders, including diabetes, hyperuric acid, hyperglycemia, non-alcoholic fatty liver, hyperlipidemia, atherosclerosis and other common diseases. We also have various metabolic disorders detection indicators and key proteins of circadian rhythm system (BMAL1, CLOCK, PER1, PER2 and PER3, etc.) related products , which can help the vast number of researchers to conduct researches on metabolic diseases and circadian rhythm.