JAK Family Decoding: Key Hubs and Potential Targets in Tumors
JAK Family Decoding: Key Hubs and Potential Targets in Tumors
1. Introduction of the JAK Family
Janus kinase (JAK) is an intracellular non-receptor tyrosine kinase that transduces cytokine-mediated signals through the JAK-signal transducer and activator of Transcription (JAK-STAT) pathway. The name "Janus" is derived from the Roman god Janus, who has two faces, because JAK has two similar phosphate transfer domains, one of which shows kinase enzyme activity, while the other motif negatively regulates the kinase activity of the former in the feedback loop.
1.1 Structure of the JAK Family
The JAK family consists of four members: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). And the JAK family is a large intracellular enzyme with approximately 1,100 amino acid residues. From the amino terminal to the carboxyl terminal, these proteins are divided into four functional domains: The amino-terminal FERM domain (mediating binding to the Box1/Box2 motifs of cytokine receptors and stabilizing the receptor-JAK complex), the SH2-like domain, the pseudo-kinase domain (JH2 domain, having no catalytic activity but regulating kinase activity through allosteric regulation), and the carboxyl-terminal kinase catalytic domain (JH1 domain, responsible for tyrosine phosphorylation, activate the downstream STAT protein). Structural differences among members affect functional specificity. For example, the FERM domain of JAK3 binds only to γ C-chain receptors due to its unique hydrophobic residue, while the pseudokinase domain of TYK2 precisely regulates kinase activity through a hydrogen bond network.
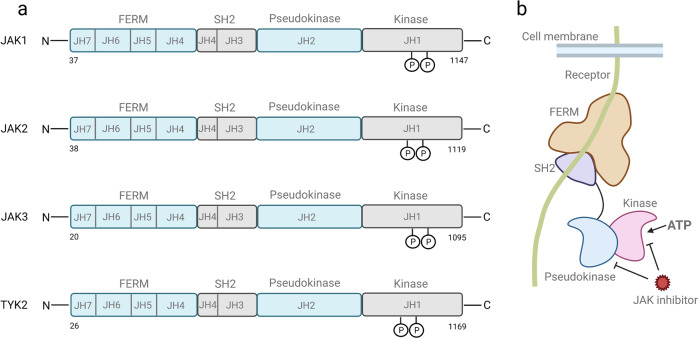
Fig.1 The structure, conserved phosphorylation sites , and inhibitors targeted sites of JAK family
(The figure is sourced from Signal Transduct Target Ther[1])
1.2 Tissues distribution of the JAK family
The distribution of JAK family proteins has significant tissue and cellular specificity, and the positioning of each member in physiological and pathological processes is closely related to its function. JAK1, JAK2 and TYK2 are widely distributed in various tissues throughout the body. Among them, JAK1 is highly expressed in immune cells (such as T cells, B cells, and macrophages) and the nervous system, mediating the signal transduction of interferon (IFN) and interleukin (IL) -6 family cytokines. JAK2 plays a dominant role in the hematopoietic system (such as the bone marrow, liver, and spleen), regulating the regulation of blood cell production by erythropoietin (EPO) and thrombopoietin (TPO). TYK2 is expressed not only in immune cells but also in skin keratinocytes and neurons, and is involved in IL-12/IL-23 signaling and neuroinflammatory regulation. In contrast, the expression of JAK3 is highly confined to the lymphatic system (such as the thymus, lymph nodes, and spleen), mainly binding to γ C-chain cytokine receptors (such as IL-2, IL-7, and IL-15 receptors), driving the development and function of T cells, NK cells, and mast cells. This distribution difference not only determines the division of labor of each member in physiological processes such as immunity and hematopoiesis, but also provides a basis for targeted therapy.
1.3 The activation method of JAK family
The activation of JAK protein depends on the dimerization and cross-phosphorylation mechanisms mediated by cytokine receptors. When cytokines bind to receptors, they induce receptor dimerization, causing the bound JAK proteins (such as JAK1/JAK2 or JAK1/TYK2) to approach each other. Through the cross-phosphorylation of tyrosine residues, the self-inhibition of the kinase domain (JH1) by the pseudo-kinase domain (JH2) is released, thereby activating catalytic activity. In addition, conformational changes in the near-membrane region of the receptor can also directly trigger allosteric activation of JAK, while phosphatases (such as SHP1) and inhibitory proteins (such as the SOCS family) negatively regulate its activity through dephosphorylation or competitive binding. Abnormal JAK activation is closely related to leukemia and autoimmune diseases. Inhibitors targeting this process achieve inhibition of the signaling pathway by binding to the ATP-catalytic pocket to block phosphate transfer.
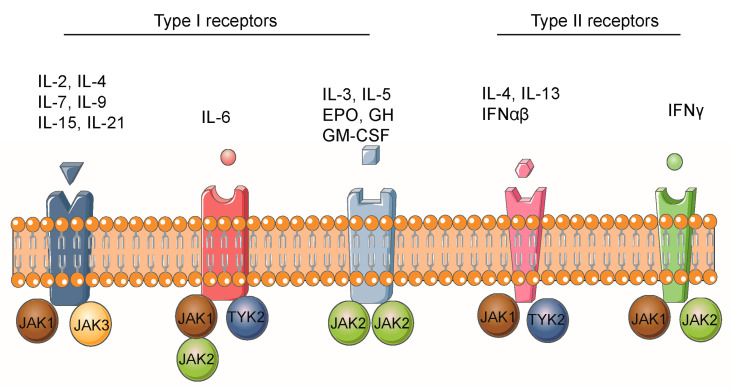
Fig.2 Cytokines activate the JAK/STAT signaling pathway
(The figure is sourced from Biomolecules[2])
2. Research related to JAKs and tumors
The JAK family, as a key intracellular non receptor tyrosine kinase, is the core hub of cytokine signaling, precisely regulating cell proliferation, differentiation, survival, apoptosis, and immune response through the JAK-STAT pathway. However, the abnormal activation, functional acquired mutations of JAKs genes, or constitutive activation of related signaling pathways have been widely confirmed to be closely related to the occurrence, development, progression, and treatment resistance of various malignant tumors. In depth research on the functional roles, dysregulation mechanisms, and interactions with the tumor microenvironment of JAK family members in tumors is not only crucial for revealing the molecular basis of tumor occurrence and development, but also provides a theoretical basis and promising new strategies for developing efficient and specific anti-tumor drugs targeting the JAK-STAT pathway.
2.1 JAKs and leukemia
The activation of tyrosine kinase genes is a common event in malignant tumors of the human hematological system. A study reported the novel somatic mutations in highly conserved residues of the JAK1 gene (T478S, V623A) in 2 separate patients with acute myeloid leukemia (AML)[3]. Compared with wild-type JAK1, JAK1T478S and JAK1V623A expression was associated with increased STAT1 activation in response to type I interferon and activation of multiple downstream signaling pathways. The research team identified a novel JAK2 acquired mutation in patients with Down syndrome (DS) with B-cell precursor acute lymphoblastic leukemia (BCP-ALL), involving a deletion of 5 amino acids within the JH2 pseudokinase domain (JAK2ΔIREED)[4]. The expression of JAK2ΔIREED in Ba/F3 cells induces constitutive activation of the JAK-STAT pathway and growth factor independent cell proliferation. Another study confirmed that JAK2 is activated in AML, and high levels of p-JAK2 are important factors for overall survival in AML patients[5]. AZ960 (a specific JAK2 kinase inhibitor) can effectively inhibit the clonal growth and induce apoptosis of freshly isolated AML cells from patients. It is reported that in patients with T-cell acute lymphoblastic leukemia (T-ALL), mitochondrial apoptotic resistance is closely related to the activation mutation of JAK3[6]. Treatment of JAK3 mutant T-ALL cells with JAK3 inhibitor Tofacitinib combined with a range of conventional chemotherapy drugs showed synergistic effects with glucocorticoids in vitro and in vivo. In addition, TYK2 mutations (P760L and G761V) were found in two T-ALL or BCP-ALL patients, leading to constitutive activation of TYK2[7]. Screening of kinase inhibitors revealed that the oncogenic TYK2 synergizes with the PI3K/AKT/mTOR and CDK4/6 pathways. When used in combination with mTOR or CDK4/6 inhibitors, the TYK2 specific inhibitor Deucravacitinib effectively reduced the cell survival rate of TYK2P760L transformed cell models and TYK2P760L mutant patient derived xenograft cells in vitro. Therefore, these studies have opened up new treatment options for JAKs driven leukemia patients.
2.2 JAKs and breast cancer
Previous studies have explored the expression and methylation level of JAKs in breast cancer, and found that the expression of JAK1 and JAK2 at the mRNA and protein levels was significantly reduced, the expression of JAK3 protein level was significantly increased, the expression of TYK2 at the mRNA level was significantly increased, but the expression of TYK2 at the protein level was significantly reduced[8]. Low transcriptional levels of JAK1, JAK2, JAK3, and TYK2 are associated with poor prognosis in breast cancer patients. In addition, the study also found that JAKs were significantly related to the immune invasion of breast cancer and the expression of multiple immune markers. A study used structure based virtual screening and molecular docking to discover six JAK2 inhibitors, among which JNN-5 was the best compound[9]. JNN-5 exhibits strong anti proliferative activity in TNBC cell lines (MDA-MB-468, MDA-MB-213, HCC70, MDA-MB-157). JNN-5 significantly reduced HUVEC migration in a dose-dependent manner. In another study, a library of benzodiazepinone derivatives targeting JAK3 kinase was synthesized to obtain anti breast cancer efficacy[10]. According to the screening, compound 5i has a good inhibitory effect on JAK3 kinase and can significantly inhibit the growth of MDA-MB-468. These studies indicate that the development of powerful inhibitors against JAKs kinase may provide help for the treatment of breast cancer.
2.3 JAKs and lung cancer
It is reported that compared with normal lung tissue, the expression level of p-JAK1 in non-small cell lung cancer (NSCLC) is significantly increased, and the positive expression of p-JAK1 indicates a poor prognosis[11]. The overall survival time of tumor patients with p-JAK1 positive expression and EGFR amplification is significantly shortened compared to tumor patients with negative ones or both features. These results demonstrate that JAK1 activation is an independent prognostic factor for NSCLC. Another study found JAK2 p.V617F somatic mutations through targeted next-generation sequencing (NGS) of NSCLC clinical cases[12]. High levels of JAK2 expression are associated with increased sensitivity to selective JAK2 inhibitors. In clinical samples of NSCLC, the increase or decrease of JAK2 due to genetic alterations is associated with a significant increase or decrease in PD-L1 expression, indicating that activation of JAK2 p.V617F mutation may confer sensitivity to JAK inhibitors and anti-PD-1 immunotherapy. In addition, administration of JAK1 inhibitor Itacitinib after anti-PD-1 immunotherapy improved immune function and anti-tumor response in mice, and achieved high response rates in phase 2 clinical trials of metastatic NSCLC[13]. Patients who were initially ineffective with anti-PD-1 immunotherapy but responded after the addition of Itacitinib improved their immune function and immune checkpoint blockade response after JAK inhibition. These results suggest that JAKs inhibition may play a role in the treatment of NSCLC by improving the efficacy of anti-PD-1 immunotherapy.
2.4 JAKs and colorectal cancer
IL-6 and downstream JAK-STAT pathways have been found to play important roles in the development of colorectal cancer (CRC). A study investigated the effect of a novel small molecule JAK inhibitor (AZD1480) on the IL-6/JAK/STAT3 pathway and its potential anti-tumor activity against human CRC cell lines (HCT116, HT29 and SW480)[14]. The results showed that AZD1480 effectively prevented constitutive and IL-6 induced phosphorylation of JAK2 and STAT-3, and exerted anti-tumor effects by reducing CRC cell proliferation and increasing cell apoptosis. Another study explored the potential of CJ14939 as a novel JAK inhibitor for the treatment of CRC[15]. CJ14939 induces CRC cell death and inhibits phosphorylation of JAK1 and STAT3. In vivo, the combination therapy of CJ14939 and oxaliplatin significantly reduced tumor growth compared to treatment with either CJ14939 or oxaliplatin alone. In addition, studies have shown that JAK-STAT signaling is activated in radiation-resistant CRC tissues and is associated with local and distant metastasis[16]. The JAK2/STAT3 signaling plays an important role in promoting tumorigenesis and radiation resistance by limiting cell apoptosis and enhancing the potential for colony formation. These studies suggest that targeted inhibition of JAKs signaling may contribute to the treatment of CRC and even radiation resistant CRC.
2.5 JAKs and other tumors
When studying how circRNA-9119 (circ9119) regulates hepatocellular carcinoma (HCC), researchers found that cancer cells isolated from HCC patients and HCC cell lines showed significantly upregulated expression of circ9119 and JAK1 compared to healthy controls and normal liver cells[17]. The administration of JAK1 inhibitor Baricitinib promotes the activation of STAT3, leading to a significant reduction in HCC cell proliferation and an increase in cell apoptosis. Another study showed that etomidate inhibits phosphorylation by competing with ATP to bind to the ATP binding pocket of JAK2, ultimately suppressing JAK2 activity. In HCC cells, it exerts its anti-tumor effect by inhibiting the JAK2/STAT3 signaling pathway[18].
The research team demonstrated that JAK1 deficiency prevents KRASG12D induced pancreatic tumor formation by using pancreatic specific knockout mice[19]. CCAAT/enhancer binding protein δ (C/EBPδ) is a biologically relevant downstream target of JAK1 signaling. Restoring the expression of C/EBP δ is sufficient to restore the growth of JAK1 deficient cancer cells in xenograft mice. The results of this study indicate that JAK1 plays an important role in inflammatory cytokines through C/EBP δ and may serve as a molecular target for the prevention and treatment of pancreatic ductal adenocarcinoma (PDAC).
Chemotherapy resistance in gastric cancer poses a significant challenge to treatment. A study evaluated the efficacy of Ruxolitinib (a JAK1/2 inhibitor) in sensitive and resistant gastric cancer cell lines[20]. Ruxolitinib induces dose-dependent mitochondrial mediated apoptosis in drug-resistant cells and enhances cytotoxicity in sensitive cells when combined with chemotherapy. In addition, treatment with Ruxolitinib significantly prolonged the overall survival of mice. These findings support further research on the clinical application of JAK1/2 inhibitors as adjuvant therapy for chemotherapy resistant gastric cancer.
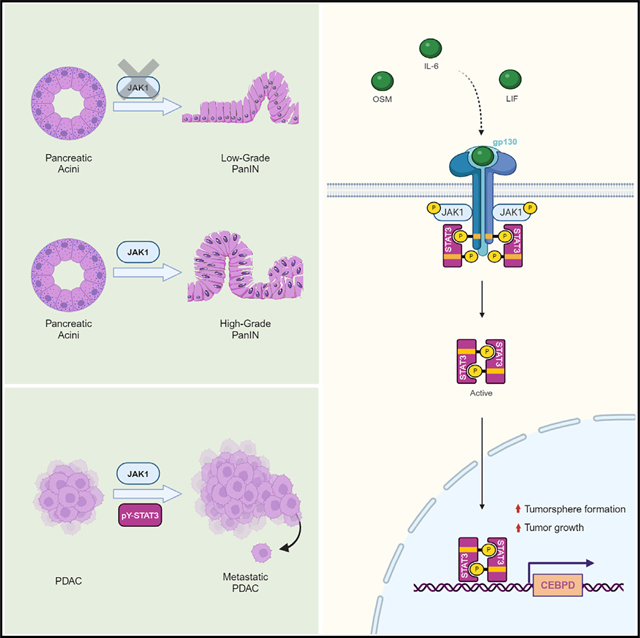
Fig.3 JAK1 is critical to the occurrence and development of pancreatic cancer
(The figure is sourced from Cell Reports[19])
Cloud-Clone supports scientific research and provides relevant detection reagent products for a wide range of scientific researchers. The core product numbers of the relevant targets are as follows:
Target | core product No. | Target | core product No. |
JAK1 | C551 | CISH | C383 |
JAK2 | F494 | IRF9 | H780 |
JAK3 | F493 | PIAS1 | B736 |
TYK2 | B595 | PIAS3 | E590 |
STAT1 | B740 | PIAS4 | E591 |
STAT2 | B796 | PTPN11 | D584 |
STAT3 | B743 | PTPN2 | D585 |
STAT4 | B739 | PTPN6 | D589 |
STAT5A | B738 | SOCS1 | H158 |
STAT5B | B727 | SOCS2 | H154 |
STAT6 | B737 | SOCS3 | B684 |
For more scientific research reagents, please visit the official website of Cloud-Clone:http://www.cloud-clone.com/
References
[1]Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):402.
[2]Liu J, Wang F, Luo F. The Role of JAK/STAT Pathway in Fibrotic Diseases: Molecular and Cellular Mechanisms. Biomolecules. 2023;13(1):119.
[3]Xiang Z, Zhao Y, Mitaksov V, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111(9):4809-4812.
[4]Malinge S, Ben-Abdelali R, Settegrana C, et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood. 2007;109(5):2202-2204.
[5]Ikezoe T, Kojima S, Furihata M, et al. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia. Int J Cancer. 2011;129(10):2512-2521.
[6]Bodaar K, Yamagata N, Barthe A, et al. JAK3 mutations and mitochondrial apoptosis resistance in T-cell acute lymphoblastic leukemia. Leukemia. 2022;36(6):1499-1507.
[7]Woess K, Macho-Maschler S, Van Ingen Schenau DS, et al. Oncogenic TYK2 P760L kinase is effectively targeted by combinatorial TYK2, mTOR and CDK4/6 kinase blockade. Haematologica. 2023;108(4):993-1005.
[8]Liu F, Wu H. Identification of Prognostic Biomarkers and Molecular Targets Among JAK Family in Breast Cancer. J Inflamm Res. 2021;14:97-114.
[9]Miao Y, Yang S, Zhang F, Li J, Zhang Y. Discovery and biological evaluation of a novel and highly potent JAK2 inhibitor for the treatment of triple negative breast cancer. J Enzyme Inhib Med Chem. 2025;40(1):2488127.
[10]Dhawan B, Alam MS, Hamid H, et al. Synthesis and biological evaluation of thiourea-tethered benzodiazepinones as anti-proliferative agents targeting JAK-3 kinase. Naunyn Schmiedebergs Arch Pharmacol. Published online March 3, 2025. doi:10.1007/s00210-025-03853-1
[11]Liu D, Huang Y, Zhang L, Liang DN, Li L. Activation of Janus kinase 1 confers poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 2017;14(4):3959-3966.
[12]Li SD, Ma M, Li H, et al. Cancer gene profiling in non-small cell lung cancers reveals activating mutations in JAK2 and JAK3 with therapeutic implications. Genome Med. 2017;9(1):89.
[13]Mathew D, Marmarelis ME, Foley C, et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. 2024;384(6702):eadf1329.
[14]Wang SW, Hu J, Guo QH, et al. AZD1480, a JAK inhibitor, inhibits cell growth and survival of colorectal cancer via modulating the JAK2/STAT3 signaling pathway. Oncol Rep. 2014;32(5):1991-1998.
[15]Hong JK, Kim DY, Shin JS, et al. CJ14939, a Novel JAK Inhibitor, Increases Oxaliplatin-induced Cell Death Through JAK/STAT Pathway in Colorectal Cancer. Anticancer Res. 2022;42(4):1813-1819.
[16]Park SY, Lee CJ, Choi JH, et al. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res. 2019;38(1):399.
[17]Yang L, Xue H, Sun Y, Zhang L, Xue F, Ge R. CircularRNA-9119 protects hepatocellular carcinoma cells from apoptosis by intercepting miR-26a/JAK1/STAT3 signaling. Cell Death Dis. 2020;11(7):605.
[18]Xu J, Zhang L, Li N, et al. Etomidate elicits anti-tumor capacity by disrupting the JAK2/STAT3 signaling pathway in hepatocellular carcinoma. Cancer Lett. 2023;552:215970.
[19]Shrestha H, Rädler PD, Dennaoui R, et al. The Janus kinase 1 is critical for pancreatic cancer initiation and progression. Cell Rep. 2024;43(5):114202.
[20]Yao Y, Zhou J, Song J, Chen C. Ruxolitinib enhances gastric cancer to chemotherapy by suppressing JAK/STAT3 and inducing mitochondrial dysfunction and oxidative stress. Immunopharmacol Immunotoxicol. 2025;47(2):263-271.
