Gut barrier and microbiota changes with glycine and branched-chain amino acid supplementation in chronic haemodialysis patients
Journal:Journal of Cachexia, Sarcopenia and Muscle
IF:12.910
On September 18, 2021, Laurence Genton and his team from the Department of Nutrition at the University Hospital of Geneva, Switzerland, published a paper titled "Gut barrier and Microbiota Changes with Glycine and colon-chain amino acid Early Supplementation in Chronic Haemodialysis patients" on Journal of Cachexia,Sarcopenia and Muscle. To assess whether glycine or branched chain amino acid (BCAA) supplementation is associated with changes in intestinal barrier and microbiota in patients with chronic hemodialysis (HD).
The ELISA Kits (ELISA Kit for Lipopolysaccharide (LPS), SEB526Hu) of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

Background: We have previously shown that glycine increases fat-free mass in chronic haemodialysis patients with features of malnutrition as compared with branched-chain amino acids (BCAAs). This multicentre randomized double-blind crossover study evaluates the impact of these amino acids on the gut barrier and microbiota.
Methods: Haemodialysis patients were included if they had plasma albumin <38 g/L or weight loss >5% of dry body weight, and daily dietary intakes <30 kcal/kg and <1 g protein/kg. They consumed glycine or BCAA (7 g twice daily) for 4 months and underwent a 1 month washout period, before crossover of supplementations. Faecal microbiota (16S rRNA gene sequencing) and immunoglobulin A (IgA), serum levels of cytokines, surrogate markers of intestinal permeability, appetite mediators, and endocannabinoids were obtained at the start and end of each supplementation. Supplementations were compared by multiple mixed linear regression models, adjusted for age, sex, month of supplementation (0 and 4 in each period), and period (Period 1: first 4 months; Period 2: last 4 months). Microbiota comparisons were performed using principal coordinate analysis and permutational multivariate analysis of variance, Shannon diversity index estimate and analysis of composition of microbiomes analysis, and Wilcoxon tests.
Results: We analysed 27 patients compliant to the supplementations. Multiple mixed linear regression models were significant only for interleukin-6 (P = 0.002), glucagon-like peptide 1 (P = 0.028), cholecystokinin (P = 0.021), and peptide YY (P = 0.002), but not for the other outcomes. The significant models did not show any impact of the type of supplementation (P < 0.05 in all models). Principal coordinate analysis and permutational multivariate analysis of variance (P = 0.0001) showed strong microbiota clustering by subject, but no effect of the amino acids. Bacterial alpha diversity and zero-radius operational taxonomic unit richness remained stable, whatever the supplementation. Lacticaseibacillus paracasei (0.030; Q1–Q3 0.008–0.078 vs. 0.004; Q1–Q3 0.001–0.070) and Bifidobacterium dentium (0.0247; Q1–Q3 0.002–0.191 vs. 0.003; Q1–Q3 0.001–0.086) significantly decreased with the BCAA supplementation.
Conclusions: The BCAA and glycine supplementations had no impact on the serum levels of cytokines, appetite mediators, intestinal permeability, endocannabinoids, or faecal IgA. Overall faecal microbiota composition and microbial diversity did not change with the glycine or BCAA supplementation but decreased the abundance of L. paracasei and B. dentium.
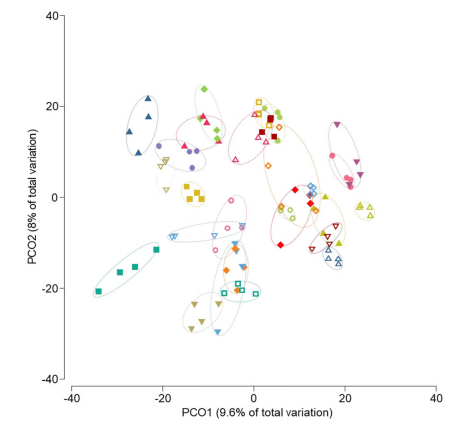
Fig 1. Principal coordinate analysis of Bray–Curtis similarity of bacterial communities
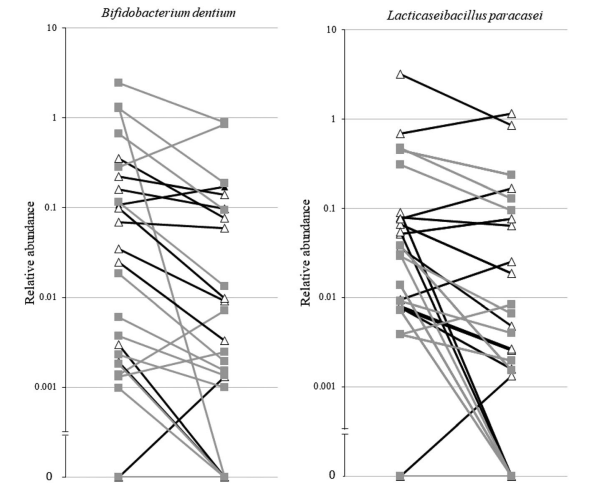
Fig 2. Line plots showing the decrease of the relative abundance of Bifidobacterium dentium and Lacticaseibacillus paracasei
