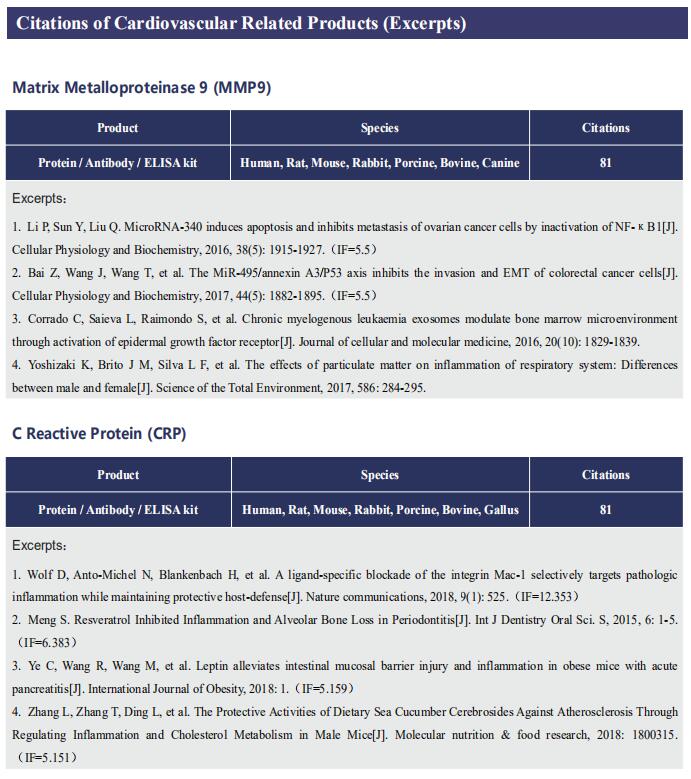
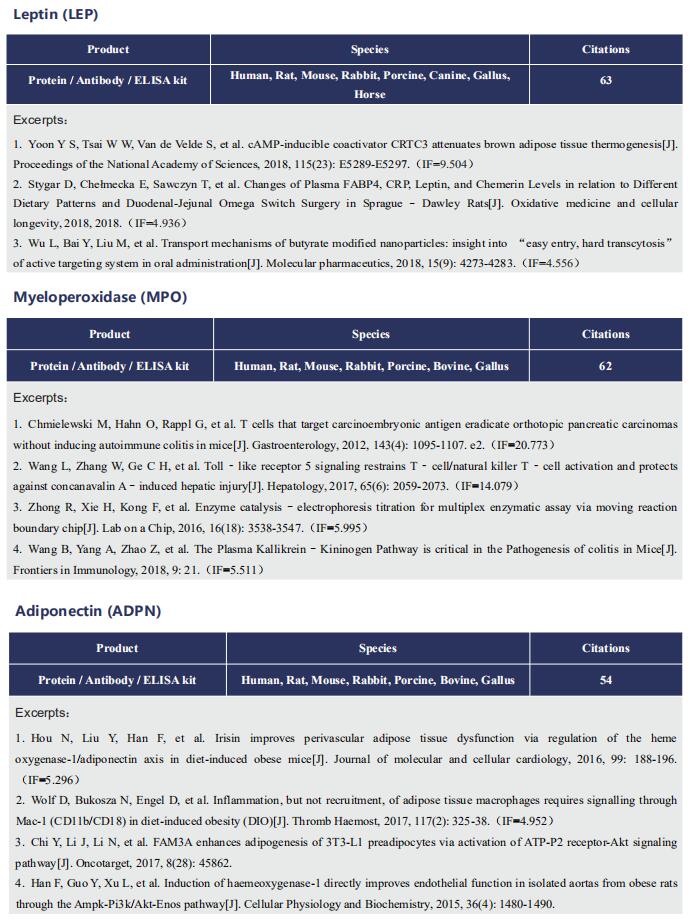
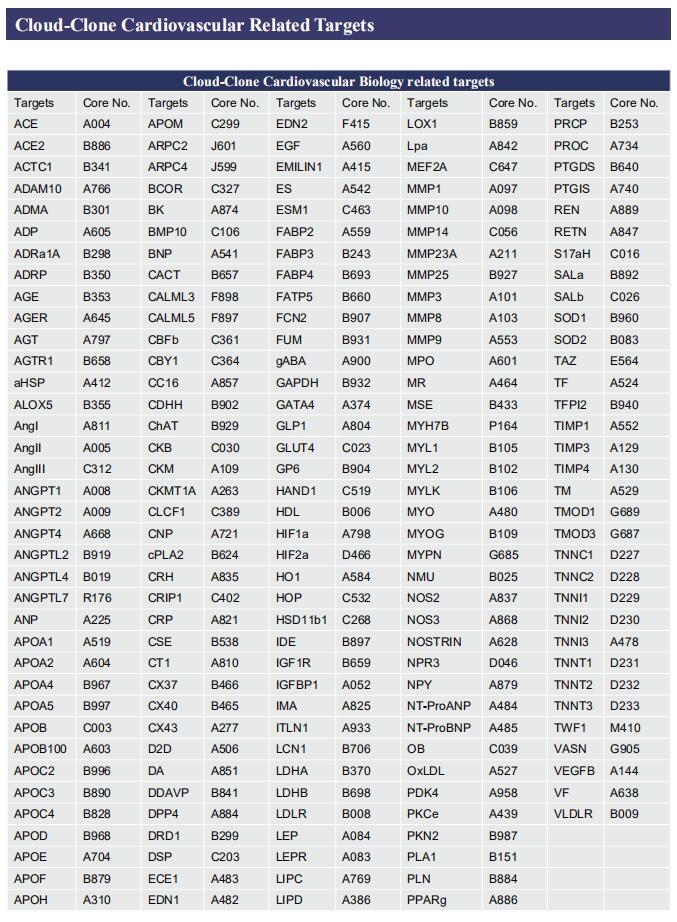
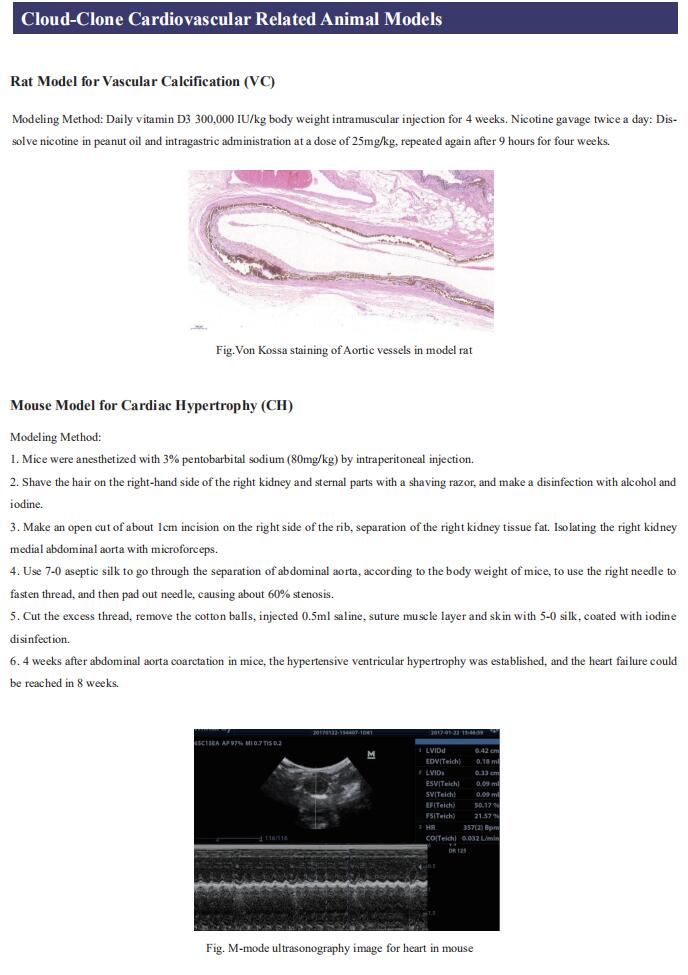
Gut microbiome and cardiovascular disease
Cardiovascular diseases (CVD), including atherosclerosis, hypertension, heart failure, atrial fibrillation and myocardial fibrosis, are associated with high morbidity and mortality. Many risk factors are widely known, such as smoking, poor dietary habits, obesity, diabetes mellitus and high cholesterol, but these cannot explain all CVD incidences. Accumulating evidence has demonstrated that gut microbiome and their metabolites play a pivotal role in the onset and progression of CVD. Recently, several studies have reported the correlation between intestinal microbes and CVD, which may provide help for the prevention and treatment of CVD.
1. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide
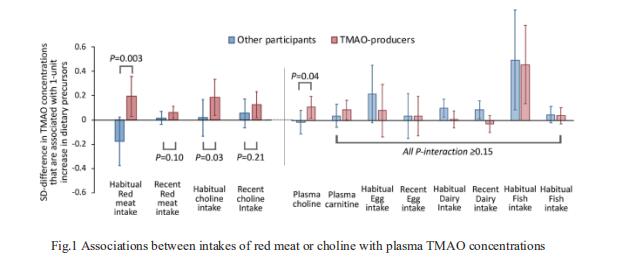
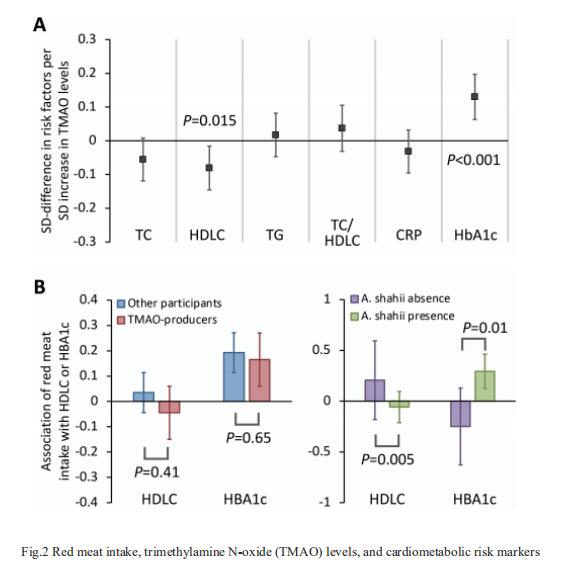
Gut-produced trimethylamine N-oxide (TMAO) is postulated as a possible link between red meat intake and poor cardiometabolic health. Qi Sun, Department of Nutrition, Harvard University TH Chan School of Public Health, and his team investigated whether gut microbiome could modify associations of dietary precursors with TMAO concentrations and cardiometabolic risk markers among free-living individuals[1]. Multivariable taxa-wide association analysis identified 10 bacterial species whose abundance was significantly associated with plasma TMAO concentrations. Higher habitual intake of red meat and choline was significantly associated with higher TMAO concentrations among participants who were microbial TMAO-producers(Fig.1). Among abundant TMAO-predicting species, Alistipes shahii significantly strengthened the positive association between red meat intake and HbA1c levels(Fig.2). They showed that a gut microbial TMAO-producer phenotype, as characterised by the identified species, modified the associations between habitual intakes of red meat/choline and plasma TMAO concentrations. In addition, specific species might also interact with red meat intake in associations with cardiometabolic risk factors. Identifying functional gut microbiome responses to dietary interventions has the potential to facilitate the development of individualised strategies for more efficient prevention of cardiometabolic diseases.
2. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism
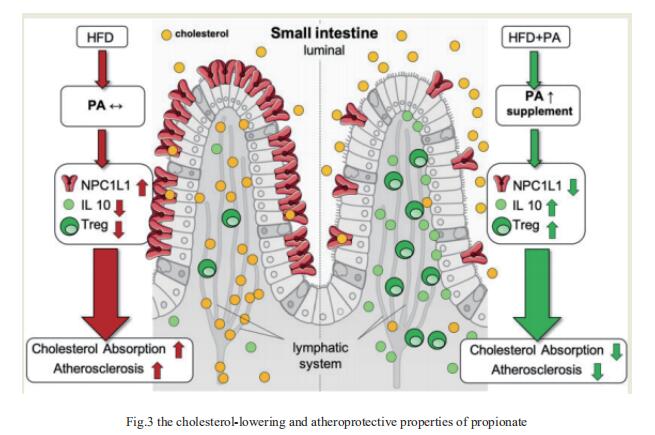
Atherosclerotic cardiovascular disease (ACVD) is a major cause of mortality and morbidity worldwide, and increased low-density lipoproteins (LDLs) play a critical role in development and progression of atherosclerosis. Arash Haghikia, Department of Cardiology, Charite´—Universita¨tsmedizin Berlin, and his team examined for the first time gut immunomodulatory effects of the microbiota-derived metabolite propionic acid (PA) on intestinal cholesterol metabolism[2]. In apolipoprotein E-/- (Apoe-/-) mice fed a high-fat diet (HFD), PA reduced intestinal cholesterol absorption and aortic atherosclerotic lesion area. Further, PA increased regulatory T-cell numbers and interleukin (IL)-10 levels in the intestinal microenvironment, which in turn suppressed the expression of Niemann-Pick C1-like 1 (Npc1l1) (Fig.3), a major intestinal cholesterol transporter. Blockade of IL-10 receptor signalling attenuated the PA-related reduction in total and LDL cholesterol and augmented atherosclerotic lesion severity in the HFD-fed Apoe-/- mice. To translate these preclinical findings to humans, they conducted a randomized, double-blinded, placebo-controlled human study. Oral supplementation with 500 mg of PA twice daily over the course of 8 weeks significantly reduced LDL, total and non-high-density lipoprotein cholesterol levels in subjects with elevated baseline LDL cholesterol levels. Those findings reveal a novel immune-mediated pathway linking the gut microbiota-derived metabolite PA with intestinal Npc1l1 expression and cholesterol homeostasis. The results highlight the gut immune system as a potential therapeutic target to control dyslipidaemia that may introduce a new avenue for prevention of ACVDs.
3. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota l-carnitine catabolism
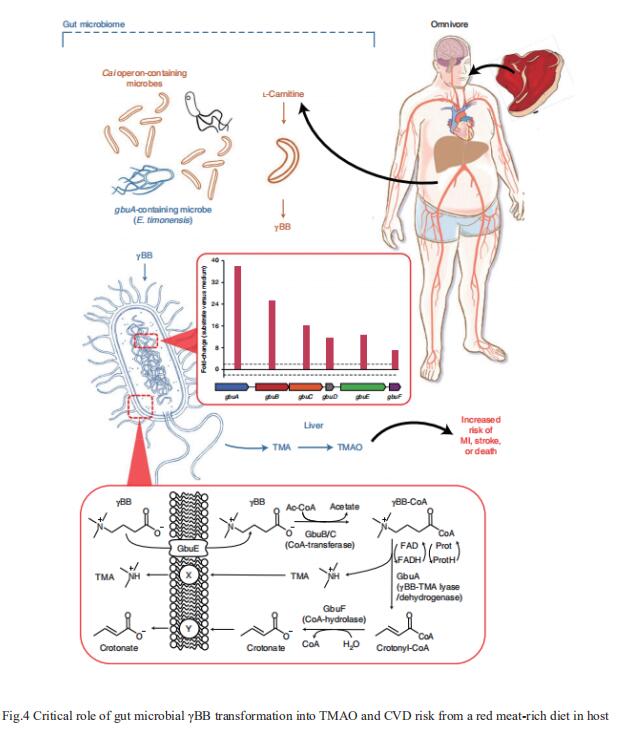
The heightened CVD risk observed among omnivores is thought to be linked, in part, to gut microbiota-dependent generation of TMAO from L-carnitine, a nutrient abundant in red meat. Gut microbial transformation of L-carnitine into trimethylamine (TMA), the precursor of TMAO, occurs via the intermediate γ-butyrobetaine (γBB). Stanley L. Hazen, Department of Cardiovascular & Metabolic Sciences, Lerner Research Institute, Cleveland Clinic, USA, and his team showed that plasma γBB levels in individuals from a clinical cohort are strongly associated with incident CVD event risks[3]. Culture of human faecal samples and microbial transplantation studies in gnotobiotic mice with defined synthetic communities showed that the introduction of Emergencia timonensis, a human gut microbe that can metabolize γBB into TMA, is sufficient to complete the carnitine →γBB → TMA transformation, elevate TMAO levels and enhance thrombosis potential in recipients after arterial injury(Fig.4). RNA-sequencing analyses of E. timonensis identified a six-gene cluster, herein named the γBB utilization (gbu) gene cluster, which is upregulated in response to γBB. Combinatorial cloning and functional studies identified four genes (gbuA, gbuB, gbuCand gbuE) that are necessary and sufficient to recapitulate the conversion of γBB to TMA when coexpressed in Escherichia coli. Finally, reanalysis of samples from a clinical, randomized diet, intervention study showed that the abundance of faecal gbuA correl iates with plasma TMAO and a red meat-rich diet. These findings reveal a microbial gene cluster thats critical to dietary carnitine →γBB → TMA → TMAO transformation in hosts and contributes to CVD risk.
References
[1]Li J, Li Y, Ivey KL, et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men [J]. Gut. 2022,71(4):724-733. (IF=23.059)
[2]Haghikia A, Zimmermann F, Schumann P, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism [J]. Eur Heart J. 2022,43(6):518-533. (IF=29.983)
[3]Buffa JA, Romano KA, Copeland MF, et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism [J]. Nat Microbiol. 2022,7(1):73-86. (IF=17.745)
Cloud-Clone can not only provide a variety of animal models of cardiovascular system diseases, covering common diseases such as hypertension, atherosclerosis, myocardial infarction, and heart failure. We also have various cardiovascular system disease detection indicators related products, which can help the majority of scientific researchers to carry out cardiovascular system diseases related research.