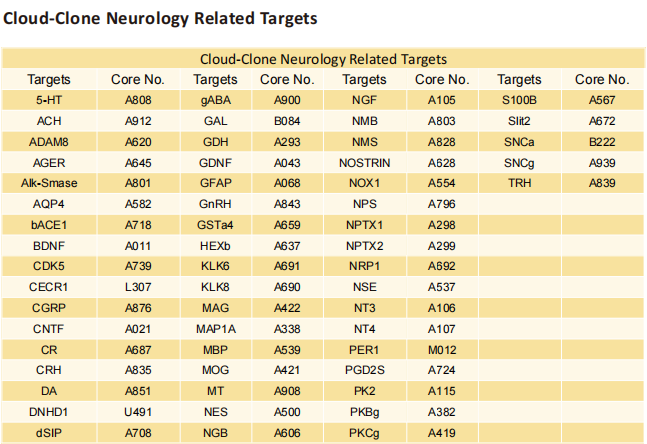
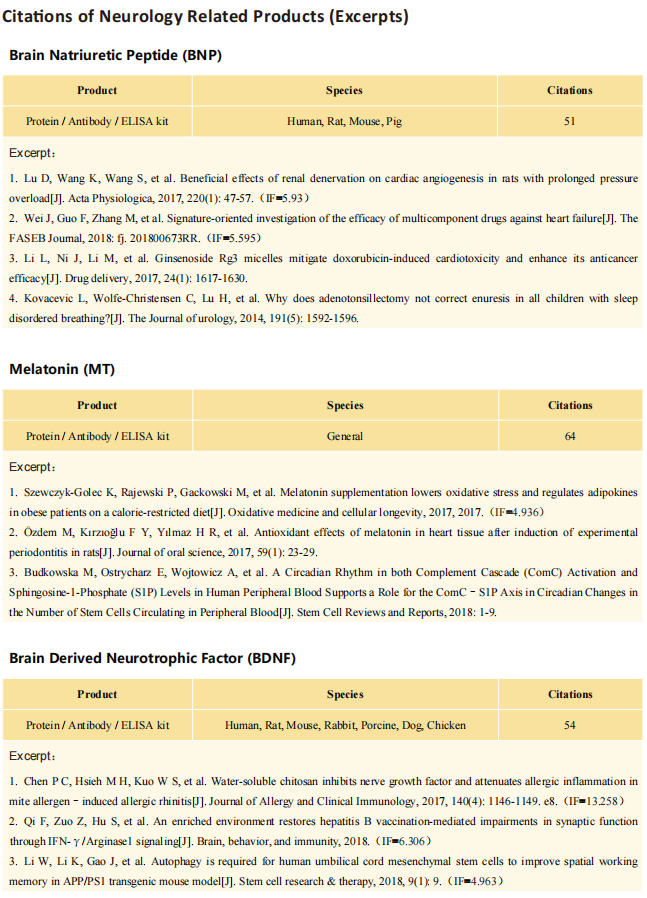
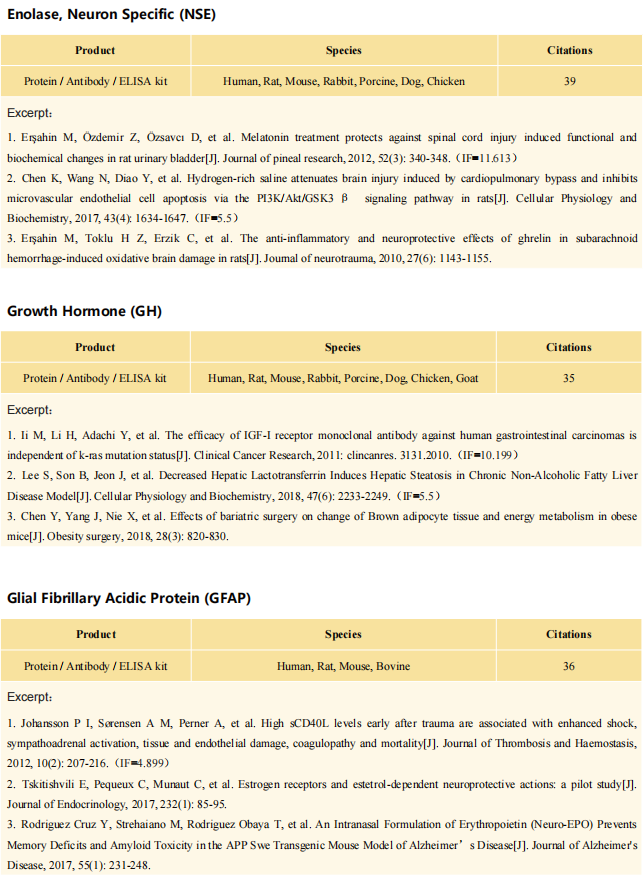
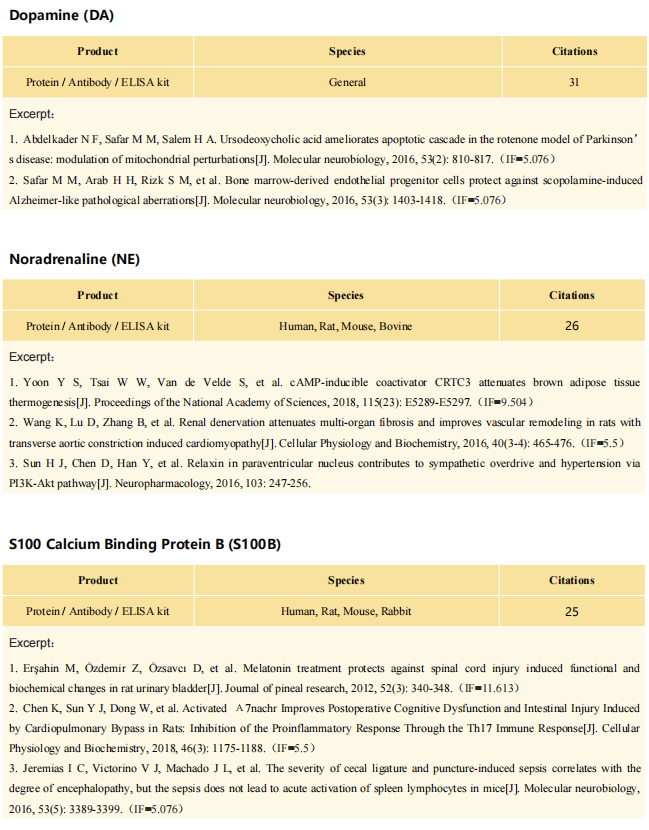
Interferon-driven brain phenotype in a mouse model of RNaseT2 deficient leukoencephalopathy
Journal:nature communications
IF:14.919
On November 11, 2021, Matthias Kettwig, Gogentine University Medical Center, Germany, and his team , published a paper titled "Interferon-driven brain phenotype in a mouse model of RNaseT2 deficient leukoencephalopathy" on Nature Communications, which suggested that RNaseT2-/- mice can be used to study central nervous system damage associated with RnaseT2 defects.

The antibody (Polyclonal Antibody to Ribonuclease T2 (RNASET2), PAA113Mu01) of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.
Infantile-onset RNaseT2 deficient leukoencephalopathy is characterised by cystic brain lesions, multifocal white matter alterations, cerebral atrophy, and severe psychomotor impairment. The phenotype is similar to congenital cytomegalovirus brain infection and overlaps with type I interferonopathies, suggesting a role for innate immunity in its pathophysiology. To date, pathophysiological studies have been hindered by the lack of mouse models recapitulating the neuroinflammatory encephalopathy found in patients. In this study, we generated Rnaset2−/− mice using CRISPR/Cas9-mediated genome editing. Rnaset2−/− mice demonstrate upregulation of interferon-stimulated genes and concurrent IFNAR1-dependent neuroinflammation, with infiltration of CD8+ effector memory T cells and inflammatory monocytes into the grey and white matter. Single nuclei RNA sequencing reveals homeostatic dysfunctions in glial cells and neurons and provide important insights into the mechanisms of hippocampal-accentuated brain atrophy and cognitive impairment. The Rnaset2−/− mice may allow the study of CNS damage associated with RNaseT2 deficiency and may be used for the investigation of potential therapies.
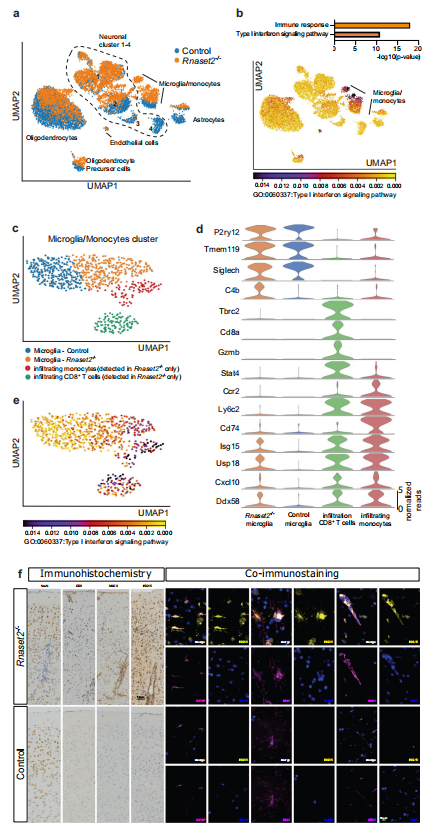
Fig. Single nuclei RNA sequencing analyses of the caudate putamen confirms type I interferon-driven neuroinflammation