New advances in blood glucose metabolism
Insulin is the key hormone for regulation of blood glucose and, generally, normoglycaemia is maintained by the balanced interplay between insulin action and insulin secretion. Importantly, the normal pancreatic β-cell can adapt to changes in insulin action—ie, a decrease in insulin action is accompanied by upregulation of insulin secretion (and vice versa). However, there is a limit to this regulation, and when β-cell dysfunction or insulin resistance occurs, glucose tolerance is impaired leading to type 2 diabetes. Recently, a number of papers have reported the related studies of blood glucose metabolism, providing help for the prevention and treatment of such metabolic diseases.
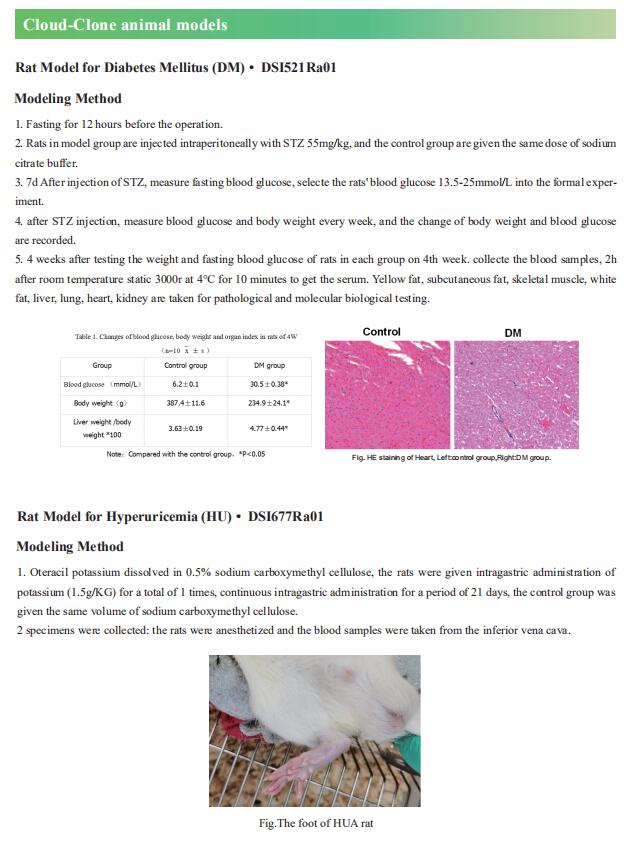
1. Glycerate from intestinal fructose metabolism induces islet cell damage and glucose intolerance
Studies that analyzed the combined effects of fat and monosaccharide consumption found that pathogenic effects of fructose are more prominent among mice fed a high-fat diet, although the mechanisms remain unknown. Xiling Shen, Department of Biomedical Engineering, Pratt School of Engineering, Duke University, USA, and his team observed that a high-fat diet altered fructose metabolism in the mouse small intestine, resulting in an enrichment of organic acid pools from the fructolysis pathway but not the citric acid cycle[1]. Fructose-derived glycerate was sustained at high levels in systemic circulation, and chronic glycerate treatment caused damage to pancreatic islet cells, especially b cells, in vivo. These data suggest that fat consumption enhances intestinal fructose metabolism and circulation of its derived glycerate (Fig.1), which can chronically damage pancreatic islet cells and lead to subsequent glucose intolerance.
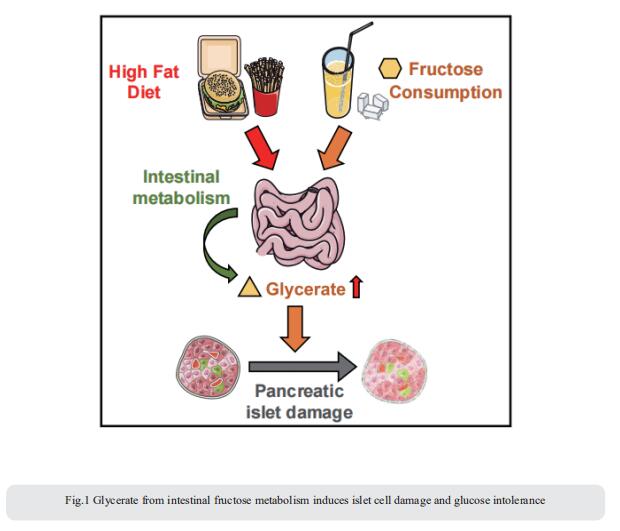
2. Palmitoylation couples insulin hypersecretion with b cell failure in diabetes
Palmitoylation, implicated in exocytosis, is reversed by acyl-protein thioesterase 1 (APT1). APT1 biology was altered in pancreatic islets from humans with type 2 diabetes. Using palmitoylation proteomics, Clay F. Semenkovich, Division of Endocrinology, Metabolism & Lipid Research, Washington University, USA, and his team identified Scamp1 as an APT1 substrate that localized to insulin secretory granules[2]. Scamp1 knockdown caused insulin hypersecretion. Expression of a mutated Scamp1 incapable of being palmitoylated in APT1-deficient cells rescued insulin hypersecretion and nutrient-induced apoptosis. High-fat-fed islet-specific APT1-knockout mice and global APT1-deficient db/db mice showed increased β cell failure. These findings suggest that APT1 is regulated in human islets and that APT1 deficiency causes insulin hypersecretion leading to β cell failure (Fig.2), modeling the evolution of some forms of human type 2 diabetes.
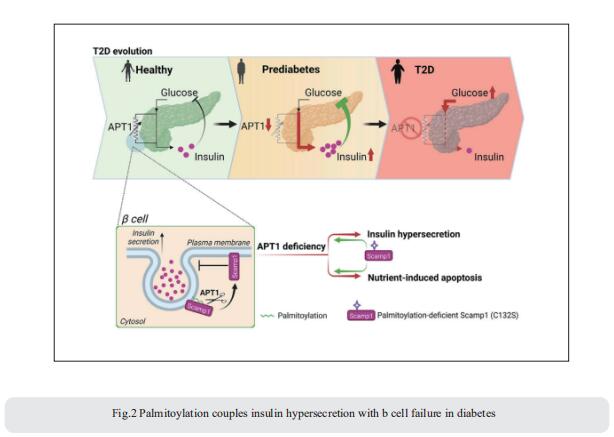
3. Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis
Altered metabolism of sphingolipids (SLs) has been linked to development of obesity and diabetes; nonetheless, the role of specific SL species in β-cell function and demise is unclear. Bengt-Frederik Belgardt, Institute for Vascular and Islet Cell Biology, German Diabetes Center (DDZ), Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Germany, and his team defined the lipid signature of diabetes-associated β-cell failure, including an imbalance of specific very-long-chain SLs and long-chain SLs[3]. β-cell-specific ablation of CerS2, the enzyme necessary for generation of very-long-chain SLs, selectively reduces insulin content, impairs insulin secretion and disturbs systemic glucose tolerance in multiple complementary models (Fig.3). In contrast, ablation of long-chain-SL-synthesizing enzymes has no effect on insulin content. By quantitatively defining the SL–protein interactome, they reveal that CerS2 ablation affects SL binding to several endoplasmic reticulum–Golgi transport proteins, including Tmed2, which they define as an endogenous regulator of the essential proinsulin processing enzyme Pcsk1. The study uncovers roles for specific SL subtypes and SL-binding proteins in β-cell function and diabetes-associated β-cell failure.
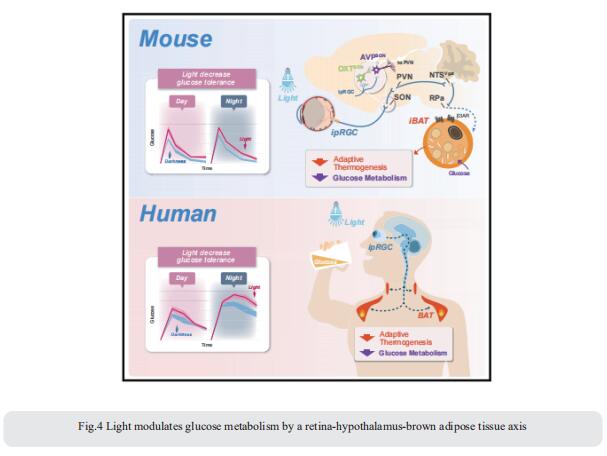
4. Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis
Artificial light is a high-risk factor for metabolic disorders. Tian Xue, CAS Key Laboratory of Brain Function and Disease, University of Science and Technology of China, China, and his team found that light can acutely decrease glucose tolerance (GT) in mice by activation of intrinsically photosensitive retinal ganglion cells (ipRGCs) innervating the hypothalamic supraoptic nucleus (SON)[4]. Vasopressin neurons in the SON project to the paraventricular nucleus, then to the GABAergic neurons in the solitary tract nucleus, and eventually to brown adipose tissue (BAT). Light activation of this neural circuit directly blocks adaptive thermogenesis in BAT, thereby decreasing GT (Fig.4). The work unveils a retina-SON-BAT axis that mediates the effect of light on glucose metabolism, which may explain the connection between artificial light and metabolic dysregulation, suggesting a potential prevention and treatment strategy for managing glucose metabolic disorders.
References
[1]Wu Y, Wong CW, Chiles EN, et al. Glycerate from intestinal fructose metabolism induces islet cell damage and glucose intolerance. Cell Metab. 2022;34(7):1042-1053.e6. (IF=31.373)
[2]Dong G, Adak S, Spyropoulos G, et al. Palmitoylation couples insulin hypersecretion with β cell failure in diabetes [published online ahead of print, 2023 Jan 6]. Cell Metab. 2023;S1550-4131(22)00549-6. (IF=31.373)
[3]Griess K, Rieck M, Müller N, et al. Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis. Nat Cell Biol. 2023;25(1):20-29. (IF=28.213)
[4]Meng JJ, Shen JW, Li G, et al. Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis. Cell. 2023;186(2):398-412.e17. (IF=66.850)
Cloud-Clone can not only provide animal models of a variety of metabolic diseases, including diabetes, high uric acid, non-alcoholic fatty liver, hyperlipidemia, atherosclerosis and other common diseases. We also have various metabolic disease detection indicators and the above-mentioned CerS2, Pcsk1 and other related products, which can help the vast number of researchers to carry out metabolic disease related research.