New advances in pancreatic cancer research
Over the past few years, the incidence of the pancreatic cancer has increased, and is expected to continue to represent a leading cause of cancer-related mortality. Most patients have no obvious symptoms during disease development and progression to advanced metastasis pancreatic, whereby tumor cells are highly invasive. Early diagnosis is difficult, and it has become one of the most deadly malignant tumors. Most patients eventually relapse, even after a potential radical treatment, the patient’s 5-year survival rate is only 2%-9%. Recently, many papers reported the new progress of pancreatic cancer research, which may help the treatment of pancreatic cancer and improve the prognosis of patients.
1. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer
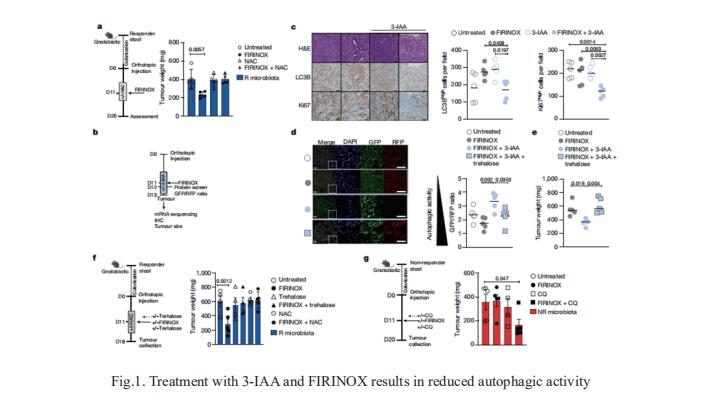
Pancreatic ductal adenocarcinoma (PDAC) is a high incidence of metastatic disease and limited responses to treatment. Less than half of all patients respond to the primary treatment for PDAC, chemotherapy, and genetic alterations alone cannot explain this. Diet is an environmental factor that can influence the response to therapies, but its role in PDAC is unclear. Nicola Gagliani, Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his team showed that the microbiota-derived tryptophan metabolite indole-3-acetic acid (3-IAA) is enriched in patients who respond to treatment by using shotgun metagenomic sequencing and metabolomic screening[1]. Faecal microbiota transplantation, short-term dietary manipulation of tryptophan and oral 3-IAA administration increase the efficacy of chemotherapy in humanized gnotobiotic mouse models of PDAC. Using a combination of loss- and gain-of-function experiments, they show that the efficacy of 3-IAA and chemotherapy is licensed by neutrophil-derived myeloperoxidase. Myeloperoxidase oxidizes 3-IAA, which in combination with chemotherapy induces a downregulation of the reactive oxygen species (ROS)-degrading enzymes glutathione peroxidase3 and glutathione peroxidase7. All of this results in the accumulation of ROS and the downregulation of autophagy in cancer cells (Fig.1), which compromises their metabolic fitness and, ultimately, their proliferation. In summary, they identified a microbiota-derived metabolite that has clinical implications in the treatment of PDAC, and provide a motivation for considering nutritional interventions during the treatment of patients with cancer.
2. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy
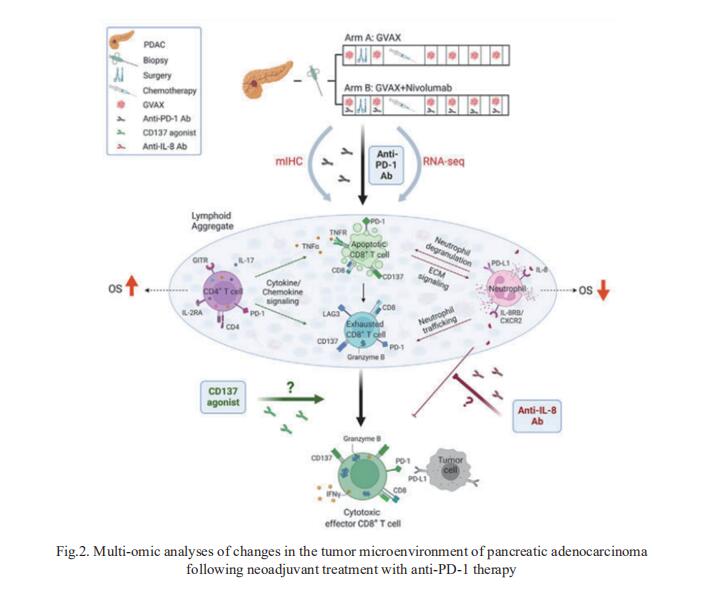
Successful pancreatic ductal adenocarcinoma (PDAC) immunotherapy necessitates optimization and maintenance of activated effector T cells (Teff). Lei Zheng, Department of Oncology, Johns Hopkins University School of Medicine, USA, and his team prospectively collected and applied multi-omic analyses to paired pre- and post-treatment PDAC specimens collected in a platform neoadjuvant study of granulocyte-macrophage colony-stimulating factor-secreting allogeneic PDAC vaccine (GVAX) vaccine ± nivolumab (anti-programmed cell death protein 1 [PD-1]) to uncover sensitivity and resistance mechanisms[2]. They showed that GVAX-induced tertiary lymphoid aggregates become immune-regulatory sites in response to GVAX + nivolumab. Higher densities of tumor-associated neutrophils (TANs) following GVAX + nivolumab portend poorer overall survival (OS). Increased T cells expressing CD137 associated with cytotoxic Teff signatures and correlated with increased OS. Bulk and single-cell RNA sequencing found that nivolumab alters CD4+ T cell chemotaxis signaling in association with CD11b+ neutrophil degranulation, and CD8+ T cell expression of CD137 was required for optimal T cell activation (Fig.2). These findings provide insights into PD-1-regulated immune pathways in PDAC that should inform more effective therapeutic combinations that include TAN regulators and T cell activators.
3. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer
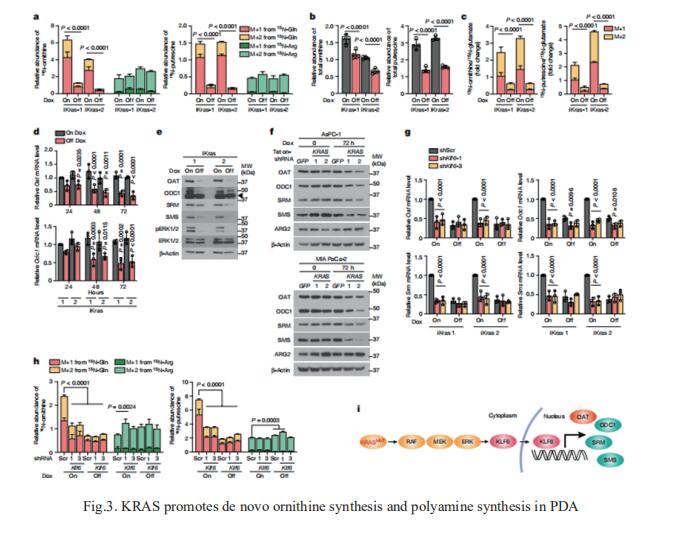
Although targeting tumour metabolism has been the focus of intense investigation for more than a decade, tumour metabolic plasticity and high risk of toxicity have limited this anticancer strategy. Nada Y. Kalaany, Division of Endocrinology, Boston Children’s Hospital, USA, and his team used genetic and pharmacological approaches in human and mouse in vitro and in vivo models to show that PDAC has a distinct dependence on de novo ornithine synthesis from glutamine[3]. They find that this process, which is mediated through ornithine aminotransferase (OAT), supports polyamine synthesis and is required for tumour growth. This directional OAT activity is usually largely restricted to infancy and contrasts with the reliance of most adult normal tissues and other cancer types on arginine-derived ornithine for polyamine synthesis. This dependency associates with arginine depletion in the PDAC tumour microenvironment and is driven by mutant KRAS. Activated KRAS induces the expression of OAT and polyamine synthesis enzymes, leading to alterations in the transcriptome and open chromatin landscape in PDAC tumour cells (Fig.3). The distinct dependence of PDAC, but not normal tissue, on OAT-mediated de novo ornithine synthesis provides an attractive therapeutic window for treating patients with pancreatic cancer with minimal toxicity.
4. Splicing Factor SRSF1 Promotes Pancreatitis and KRASG12D-Mediated Pancreatic Cancer
Dysregulated RNA splicing factors have been widely reported in tumorigenesis, but their involvement in pancreatitis and PDAC is not well understood. Adrian R. Krainer, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, United States, and his team reported that the splicing factor SRSF1 is highly expressed in pancreatitis, PDAC precursor lesions, and tumors[4]. Increased SRSF1 is sufficient to induce pancreatitis and accelerate KRASG12D-mediated PDAC. Mechanistically, SRSF1 activates MAPK signaling—partly by upregulating interleukin 1 receptor type 1 (IL1R1) through alternative-splicing-regulated mRNA stability (Fig.4). Additionally, SRSF1 protein is destabilized through a negative feedback mechanism in phenotypically normal epithelial cells expressing KRASG12D in mouse pancreas, and in pancreas organoids acutely expressing KRASG12D, buffering MAPK signaling and maintaining pancreas cell homeostasis. This negative-feedback regulation of SRSF1 is overcome by hyperactive MYC, facilitating PDAC tumorigenesis. These findings implicate SRSF1 in the etiology of pancreatitis and PDAC, and point to SRSF1-misregulated alternative splicing as a potential therapeutic target.

References
[1]Tintelnot J, Xu Y, Lesker TR, et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer [J]. Nature. 2023;615(7950):168-174. (IF=69.504)
[2]Li K, Tandurella JA, Gai J, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy [J]. Cancer Cell. 2022;40(11):1374-1391.e7. (IF=38.585)
[3]Lee MS, Dennis C, Naqvi I, et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer [J]. Nature. 2023;616(7956):339-347. (IF=69.504)
[4]Wan L, Lin KT, Rahman MA, et al. Splicing Factor SRSF1 Promotes Pancreatitis and KRASG12D-Mediated Pancreatic Cancer [J]. Cancer Discov. 2023;CD-22-1013. (IF=38.272)
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators and the above-mentioned IAA, OAT, SRSF1, IL1R1, MAPK, MYC and other related products, which can help scientific researchers to conduct cancer-related research.

