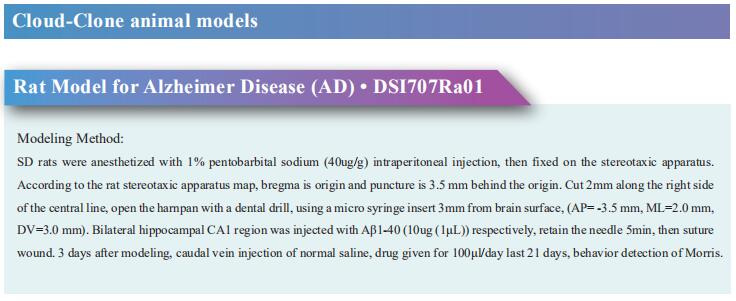
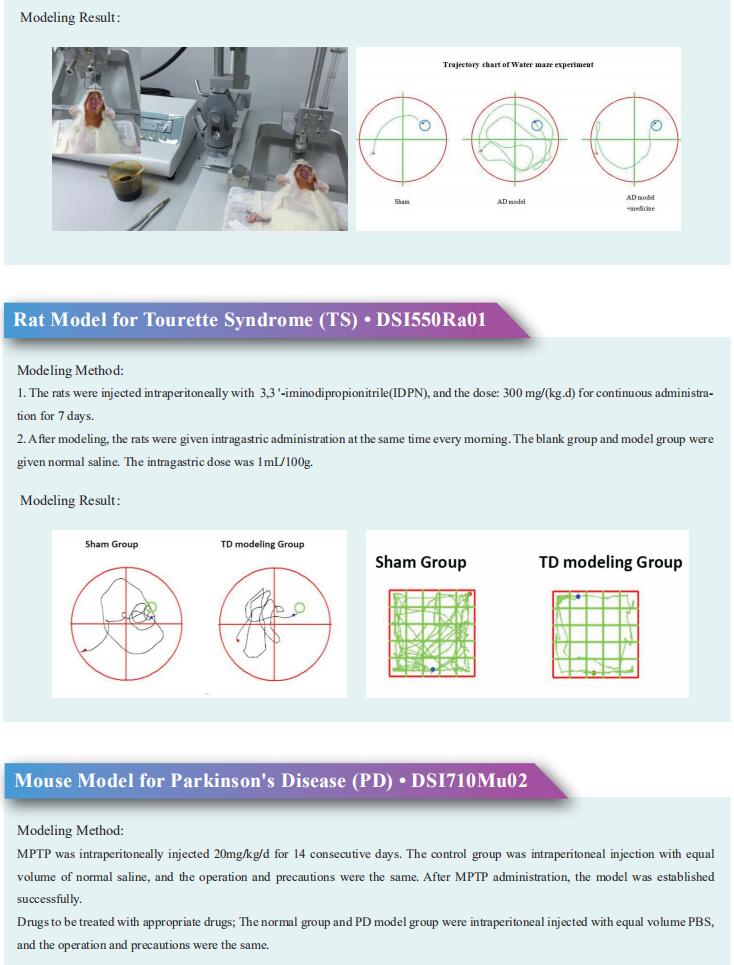
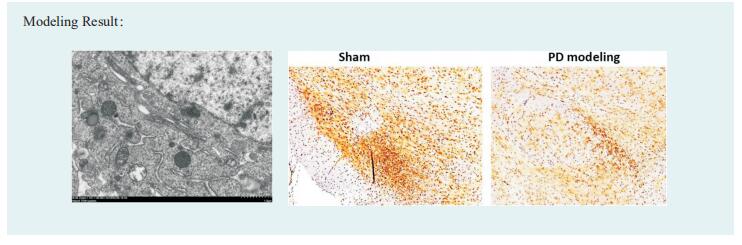
New discoveries in epilepsy research
Epilepsy occurs when there is abnormal synchronous neuronal firing in a section of the brain, or throughout the entirety of the brain. In children, the most common causes of epilepsy are genetic, injury due to perinatal insults, and malformations of cortical development. In adults without a genetic predisposition to epilepsy, common etiologies for seizures include encephalitis/meningitis, traumatic brain injury, and brain tumors. In elderly patients, epilepsy is usually the result of primary neurodegenerative disorders, head trauma, and brain tumors. Recently, some papers have reported epilepsy related research, which may provide help for the prevention and treatment of epilepsy.
1. Comprehensive multi-omic profiling of somatic mutations in malformations of cortical development
Malformations of cortical development (MCD) are neurological conditions involving focal disruptions of cortical architecture and cellular organization that arise during embryogenesis, largely from somatic mosaic mutations, and cause intractable epilepsy. Joseph G. Gleeson, Department of Neurosciences, University of California San Diego, USA, and his team reported a genetic landscape from 283 brain resections, identifying 69 mutated genes through intensive profling of somatic mutations, combining whole-exome and targeted-amplicon sequencing with functional validation including in utero electroporation of mice and single-nucleus RNA sequencing[1]. Genotype-phenotype correlation analysis elucidated specifc MCD gene sets associated with distinct pathophysiological and clinical phenotypes. The unique single-cell level spatiotemporal expression patterns of mutated genes in control and patient brains indicate critical roles in excitatory neurogenic pools during brain development and in promoting neuronal hyperexcitability after birth (Fig.1). These findings could lead to new molecular classifications and diagnostic strategies for MCD and ultimately to personalized therapies for epilepsy.
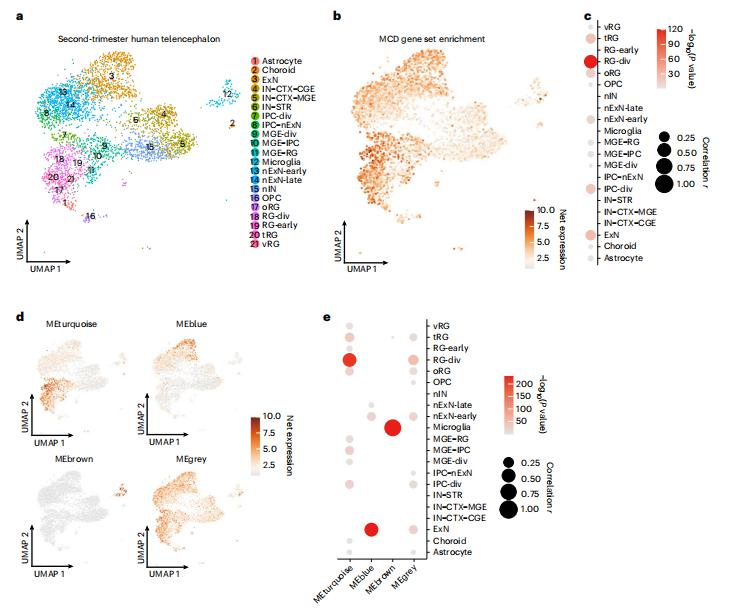
Fig.1 Single-nucleus transcriptomes reveal MCD gene enrichment in radial glia and excitatory neurons in the developing human cortex
2. Metabolic modulation of synaptic failure and thalamocortical hypersynchronization with preserved consciousness in G1D
Individuals with glucose transporter type I deficiency (G1D) habitually experience nutrient-responsive epilepsy associated with decreased brain glucose. However, the mechanistic association between blood glucose concentration and brain excitability in the context of G1D remains to be elucidated. Juan M. Pascual, Rare Brain Disorders Program, Department of Neurology, University of Texas Southwestern Medical Center, USA, and his team used 18F-deoxyglucose positron emission tomography (18F-DG-PET) and mass spectrometry on the G1D mice, which showed decreased brain glucose and glycogen, but preserved tricarboxylic acid cycle intermediates, indicating no overall energy metabolism failure[2]. In brain slices from these animals, synaptic inhibition of cortical pyramidal neurons and thalamic relay neurons was decreased (Fig.2), and neuronal disinhibition was mitigated by metabolic sources of carbon; tonic-clonic seizures were also suppressed by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor inhibition. These results pose G1D as a thalamocortical synaptic disinhibition disease associated with increased glucose-dependent neuronal excitability, possibly in relation to reduced glycogen.
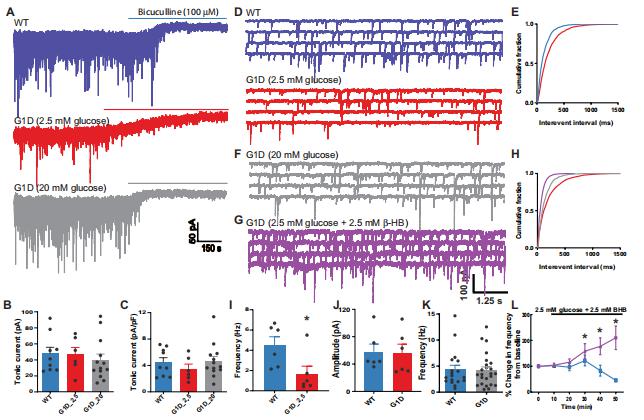
Fig.2 Inhibitory neurotransmission onto ventrobasal thalamus relay neurons in G1D
3. Treatment of epilepsy using a targeted p38γ kinase gene therapy
Previous research has shown that site-specific phosphorylation of tau at threonine 205 (T205) by the kinase p38γ was shown to disengage tau from toxic pathways, serving a neuroprotective function in Alzheimer’s disease. To probe the mechanistic relevance of this postsynaptic tau/p38γ interaction in epilepsy, Lars M. Ittner, Dementia Research Centre, Macquarie Medical School, Faculty of Medicine, Health and Human Sciences, Macquarie University, Australia, and his team generated two epilepsy mouse models: a genetic model of the infantile-onset epileptic encephalopathy Dravet syndrome (DS) and a chemically induced epilepsy mouse model to study acute and chronic seizure effects[3]. Using a viral-mediated gene delivery approach in different mouse models of epilepsy, they show that p38γ activity–enhancing treatment reduces seizure susceptibility, restores neuronal firing patterns, reduces behavioral deficits, and ameliorates epilepsy-induced deaths. Furthermore, they show that p38γ-mediated phosphorylation of tau at T205 is essential for this protection in epilepsy (Fig.3), as a lack of this critical interaction reinstates pathological features and accelerates epilepsy in vivo. Hence, their work provides a scope to harness p38γ as a future therapy applicable to acute neurological conditions.
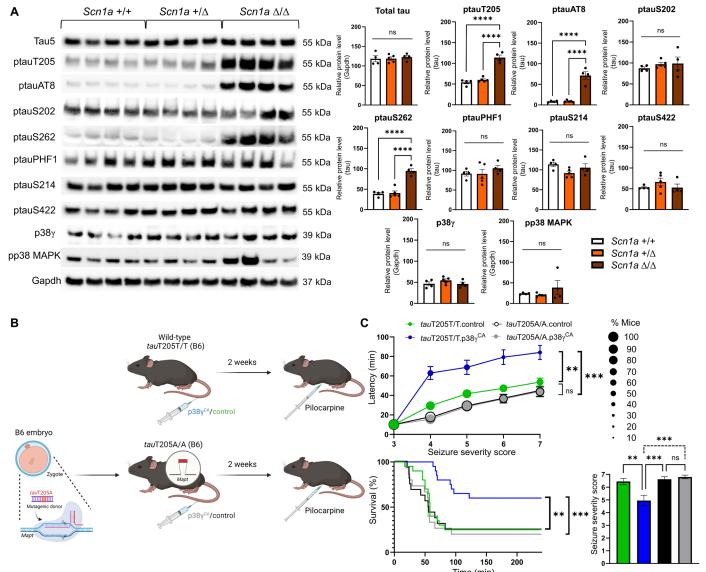
Fig.3 Tau T205 is a protective site in epilepsy
References
[1]Chung C, Yang X, Bae T, et al. Comprehensive multi-omic profiling of somatic mutations in malformations of cortical development. Nat Genet. 2023;55(2):209-220. (IF=41.307)
[2]Rajasekaran K, Ma Q, Good LB, et al. Metabolic modulation of synaptic failure and thalamocortical hypersynchronization with preserved consciousness in Glut1 deficiency. Sci Transl Med. 2022;14(665):eabn2956. (IF=19.319)
[3]Morey N, Przybyla M, van der Hoven J, et al. Treatment of epilepsy using a targeted p38γ kinase gene therapy. Sci Adv. 2022;8(48):eadd2577. (IF=14.957)
Cloud-Clone can not only provide animal models of various nervous system diseases, including epilepsy, Alzheimer's disease, schizophrenia, behavioral despair depression, chronic stress depression, anxiety, etc., covering the research of common neurological diseases. We can also provide the detection products of indicators related to nervous system diseases and the above AMPA receptor related products, which can help the majority of scientific researchers to carry out research related to nervous system diseases.