New discovery of drug resistance mechanism in tumor cells
Conventional chemotherapy and targeted therapy constitute an important part of first-line therapy for cancer management. Most patients with early-stage tumors achieve complete or partial response after chemotherapy and targeted therapy, while patients with advanced tumors often have poor treatment outcomes. Cancer cells evade the cytotoxicity of chemotherapy and targeted therapy through multifactorial intrinsic and acquired drug resistance mechanisms, and drug resistance is the main cause of chemotherapy failure.
Study on drug resistance mechanism of tumor cells
The development of drug resistance is a major challenge in cancer treatment. Adaptation in cancer therapy promotes drug resistance by multiple mechanisms, including changes in anticancer drug targets, activation of parallel bypass signaling pathways, changes in tumor microenvironment, REPAIR of DNA damage, epithelial-to-mesenchymal transformation (EMT), and changes in cellular pharmacology. The study of these resistance mechanisms may become therapeutic targets for drug-resistant cancers and help improve cancer resistance outcomes.
1. FGFR inhibitor mediated dismissal of SWI/SNF complexes from YAP-dependent enhancers induces adaptive therapeutic resistance
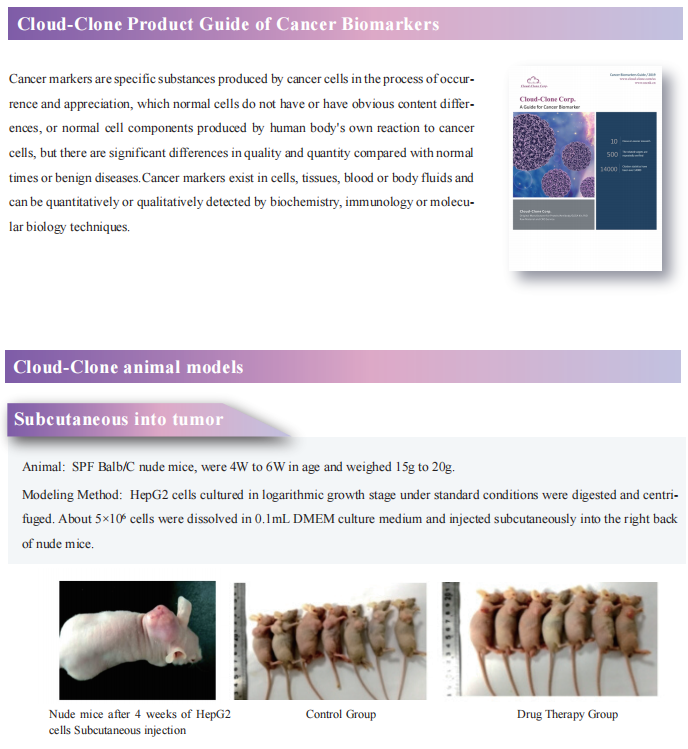
Fibroblast growth factor receptor (FGFR) inhibitors in triple negative breast cancer (TNBC) effectively block tumor cell proliferation. However, adaptive or intrinsic resistance is a common problem limiting therapeutic efficacy. Myles Brown, Dana-Farber Cancer Institute, Harvard Medical School, and his team reported an epigenetic mechanism leading to the adaptive resistance of TNBC to FGFR inhibitors[1]. They identified mTOR and YAP loss as sensitizers of FGFR inhibition, while ARID1A or BRG1 depletion can engender drug resistance. And they demonstrated that adaptive resistance to FGFR inhibition occurs via inhibition of SWI/SNF-regulated epigenetic state, resulting in reactivation of YAP dependent enhancers that in turn promote the amino acid transport that is sensed by the mTORC1 pathway leading to cell growth(Fig.1). This FGFR inhibitor activated feedback loop that limits the efficacy of these inhibitors can be reversed by mTORC1 inhibition.
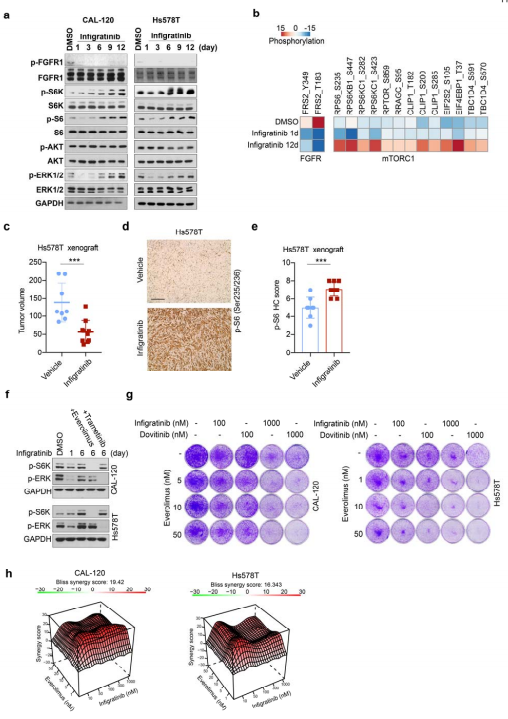
Fig.1 Reactivation of mTORC1 signaling is required for adaptive resistance to FGFR inhibitors
2. Cancer cell-derived exosomal circUSP7 induces CD8+ T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC
In recent years, an increasing number of studies have reported that exosomes perform important biological functions in the body, especially in the occurrence and development of tumours and the presence of circRNAs. Yong-Bing Wu of , the Second Afliated Hospital of Nanchang University and his team found that circUSP7 was predominantly secreted by NSCLC cells in an exosomal manner, and circUSP7 inhibits IFN-γ, TNF-α, Granzyme-B and Perforin secretion by CD8+ T cells[2]. Furthermore, circUSP7 inhibits CD8+ T cell function by upregulating the expression of Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2) via sponging miR-934. And they also showed that circUSP7 may promote resistance to anti-PD1 immunotherapy in NSCLC patients(Fig.2).
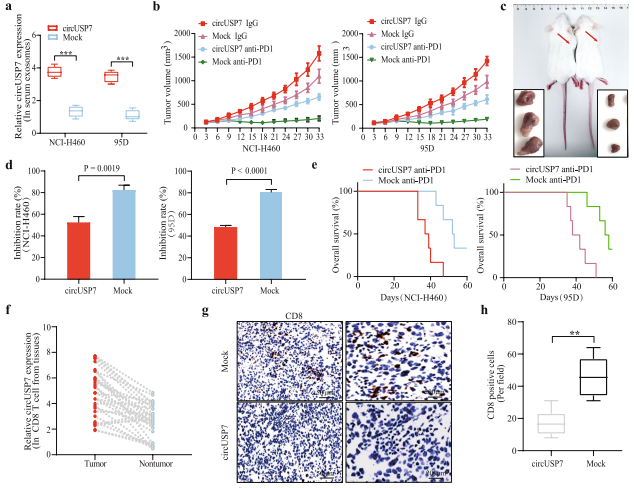
Fig.2 CircUSP7 promotes NSCLC progression in a CD8+ T cell-dependent manner and leads to resistance to anti-PD1 therapy
3. Inhibition of USP28 overcomes Cisplatin-resistance of squamous tumors by suppression of the Fanconi anemia pathway
Squamous cell carcinomas (SCC) frequently have an exceptionally high mutational burden. As consequence, they rapidly develop resistance to platinum-based chemotherapy.ΔNp63 contributes to the resistance of SCC towards platin-based chemotherapy due to its ability to regulate the expression of DNA-damage response genes. Markus E. Diefenbacher, Department of Biochemistry and Molecular Biology, University of Würzburg, and his team reported that USP28 maintains via ΔNp63 the genomic integrity of SCCs during chemotherapy with cisplatin and that targeting USP28 sensitizes ΔNp63 positive SCC to chemotherapy[3]. ΔNp63 enhances the expression of the fanconi anemia gene, which contributes to recombinant DNA repair and cisplatin resistance(Fig.3). Targeting the USP28-ΔNp63 axis in SCC tones down the FA-DDR pathway, thereby sensitizing SCC to Cisplatin treatment.
Fig.3 Inhibition of USP28 activity can relieve fanconi anemia -DNA damage repair signaling pathway in vivo, making tumor cells sensitive to cisplatin
4. The Q61H mutation decouples KRAS from upstream regulation and renders cancer cells resistant to SHP2 inhibitors
Cancer cells bearing distinct KRAS mutations exhibit variable sensitivity to SHP2 inhibitors (SHP2i). Mitsuhiko Ikura, Canada Princess Margaret Cancer Centre, and his team showed that cells harboring KRAS Q61H are uniquely resistant to SHP2i, and investigate the underlying mechanisms using biophysics, molecular dynamics, and cell-based approaches[4]. Q61H mutation impairs intrinsic and GAP-mediated GTP hydrolysis, and impedes activation by SOS1. SHP2 can stimulate KRAS signaling by modulating GEF/ GAP activities and dephosphorylating KRAS. Phosphorylation of wild-type and Gly12-mutant KRAS, which are associated with sensitivity to SHP2i, confers resistance to regulation by GAP and GEF activities and impairs binding to RAF, whereas the near-complete GAP/GEF-resistance of KRAS Q61H remains unaltered, and highaffinity RAF interaction is retained(Fig.4). So that SHP2i could not regulate the signal transduction of Q61H mutant and was resistant to drugs.
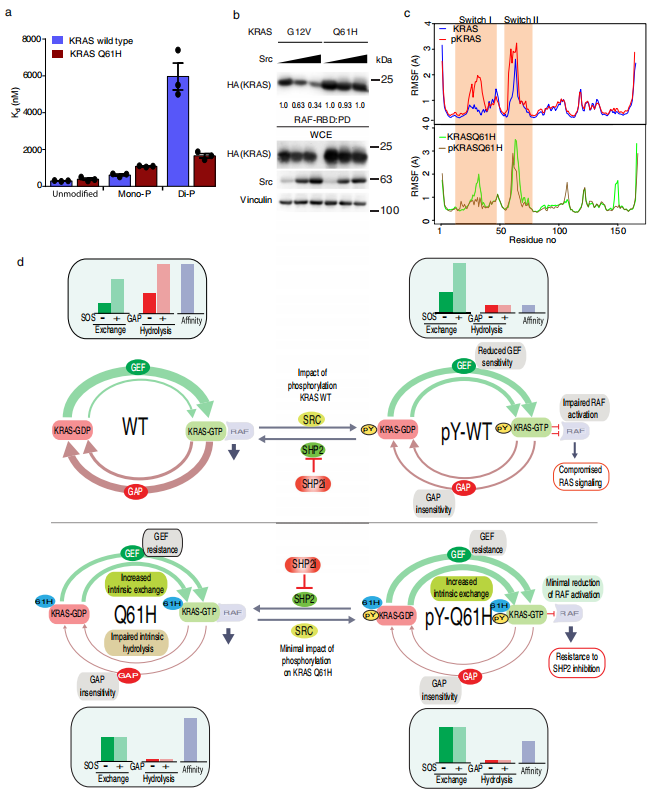
Fig.4 KRAS Q61H is resistant to regulation of RAF-RBD binding by tyrosyl phosphorylation
References
[1]Li Y, Qiu X, Wang X, et al. FGFR-inhibitor-mediated dismissal of SWI/SNF complexes from YAP-dependent enhancers induces adaptive therapeutic resistance [J]. Nat Cell Biol. 2021, 23(11):1187-1198. (IF=28.824)
[2]Chen SW, Zhu SQ, Pei X, et al. Cancer cell-derived exosomal circUSP7 induces CD8+ T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC [J]. Mol Cancer. 2021, 20(1):144. (IF=27.401)
[3]Prieto-Garcia C, Hartmann O, Reissland M, et al. Inhibition of USP28 overcomes Cisplatin-resistance of squamous tumors by suppression of the Fanconi anemia pathway [J]. Cell Death Differ. 2021;10.1038/s41418-021-00875-z. (IF=15.828)
[4]Gebregiworgis T, Kano Y, St-Germain J, et al. The Q61H mutation decouples KRAS from upstream regulation and renders cancer cells resistant to SHP2 inhibitors [J]. Nat Commun. 2021;12(1):6274. (IF=14.919)
Cloud-Clone not only provide a variety of experimental tumor animal models, including tumor transplantation animal model, spontaneous tumor animal model, induced tumor animal model, tumor metastasis animal model, covering common tumor research. It also has various cancer detection indicators related products, which can help the majority of scientific researchers to conduct cancer drug resistance related research.