New discovery of pancreatitis disease mechanism
Pancreatitis is a gastrointestinal disorder with considerable morbidity and mortality. Acute pancreatitis, recurrent acute pancreatitis, and chronic pancreatitis form a disease continuum. Some factors causing these diseases include alcohol, bile acids, and ductal obstruction, where they induce the inflammatory response that promotes their pathogenesis. Common cellular events underlying the pathogenesis of acute pancreatitis include increased oxidative stress, premature intra-acinar trypsinogen activation, activated calcium signaling, mitochondrial dysfunction, endoplasmic reticulum stress, and autophagy impairment. Recently, several articles have reported new insights into the mechanism of pancreatitis, which can be used for disease prevention and treatment.
1. Alcohol predisposes obese mice to acute pancreatitis via visceral adipocyte lipolysis
Long-term studies have shown that alcohol increases the risk of hereditary susceptibility to chronic pancreatitis. However, no study investigated the synergistic effect between obesity and alcohol excess on acute pancreatitis (AP) development. Wei Huang, Department of Integrated Traditional Chinese and Western Medicine, Sichuan Provincial Pancreatitis Centre and West China-Liverpool Biomedical Research Centre, West China Hospital, Sichuan University, China, and his team reported the combination of acute alcohol intake and obesity causes AP with multiorgan injury (MOI) in mice, mediated by visceral adipocyte tissue (VAT) lipolysis[1]. Acute ethanol administration in obese mice induced significant increases in pancreatic histopathology scores, elevated circulating pancreatic enzymes, pancreatic and lung myeloperoxidase, and serum interleukin-6 levels. In contrast, acute ethanol administration in lean mice caused only mild pancreatic oedema without discernable pancreatic necrosis or elevations of MOI indices. Mechanism analysis showed that obesity and alcohol act synergistically in the pathogenesis of onset and development of MOI in obese alcoholic AP through induction of adipose triglyceride lipase (ATGL)-mediated VAT lipolysis(Fig.1). These results suggest that ATGL may be a potential target for the prevention and treatment of obese alcoholic AP.
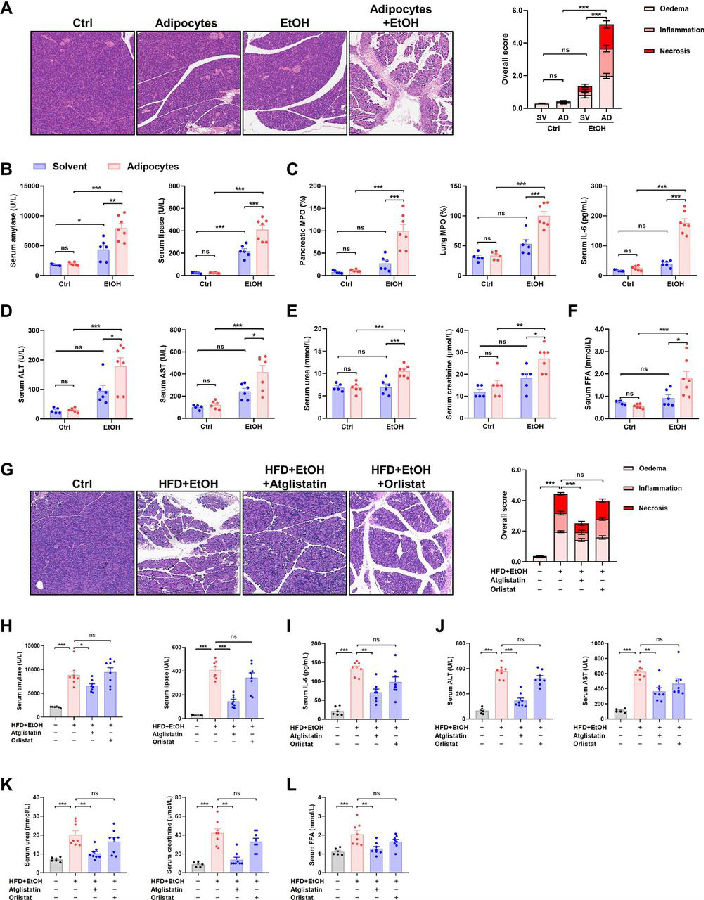
Fig.1 Obesity and acute alcohol intake cause AP through visceral adipocyte lipolysis mediated by ATGL
2. Estrogen-Related Receptor g Maintains Pancreatic Acinar Cell Function and Identity by Regulating Cellular Metabolism
Mitochondrial dysfunction disrupts the synthesis and secretion of digestive enzymes in pancreatic acinar cells and plays a primary role in the etiology of exocrine pancreas disorders. Jae Myoung Suh, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Korea, and his team aimed to elucidate the function of estrogen-related receptor g (ERRg) in pancreatic acinar cell mitochondrial homeostasis and energy production[2]. Blocking ERRg function in mice by genetic deletion or inverse agonist treatment results in striking pancreatitis-like phenotypes accompanied by inflammation, fibrosis, and cell death. Mechanistically, loss of ERRg in primary acini abrogates messenger RNA expression and protein levels of mitochondrial oxidative phosphorylation complex genes, resulting in defective acinar cell energetics. Mitochondrial dysfunction due to ERRg deletion further triggers autophagy dysfunction, endoplasmic reticulum stress, and production of reactive oxygen species, ultimately leading to cell death (Fig.2). ERRg expression was significantly reduced in patients with chronic pancreatitis compared with normal subjects. Furthermore, candidate locus region genetic association studies revealed multiple single nucleotide variants for ERRg that are associated with chronic pancreatitis. These findings advance the understanding of the molecular programs regulating acinar cell metabolism and have therapeutic implications for relevant disorders, such as pancreatitis and pancreatic cancers, as ERRg lies within a pharmacologically accessible niche.
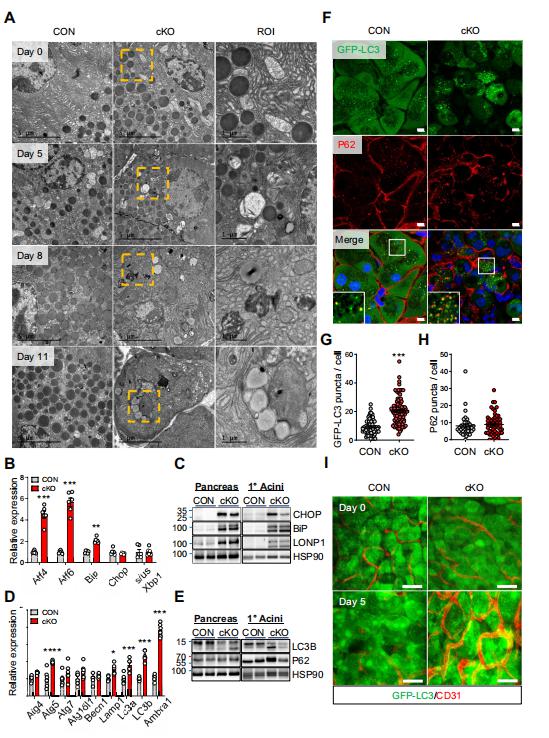
Fig.2 Loss of ERRg results in ER stress and autophagy in pancreatic acinar cells
3. Wild-Type Human PRSS2 and PRSS1R122H Cooperatively Initiate Spontaneous Hereditary Pancreatitis in Transgenic Mice
Premature digestive enzyme activation in the pancreas is a major cause of pancreatitis. The gain-of-function trypsinogen PRSS1 gene mutations are linked to the development of human hereditary pancreatitis. PRSS1R122H—the most common mutation in human hereditary pancreatitis—the many attempts to develop a genetic mouse model for hereditary pancreatitis by transgenic expression of human PRSS1R122H have yielded limited success. None of these models showed evidence of spontaneous pancreatitis.Baoan Ji, Department of Cancer Biology, Mayo Clinic, USA, and his team found that increased wild-type PRSS2, another major isoform of human trypsinogens, is also detrimental in pancreatitis[3]. They generated new transgenic mouse lines using a human bacterial artificial chromosome harboring full-length human PRSS1 and PRSS2 genes with intact promoter and introns. One mouse line expresses wild-type PRSS1 and PRSS2 (PRSS1-PRSS2 mice). In the other line, a point mutation was introduced in the PRSS1 gene via GalKmediated recombineering technology to express both mutant PRSS1R122H and wild-type PRSS2 (PRSS1R122H-PRSS2 mice). Compared with wild-type PRSS1, mutant PRSS1R122H stimulates the development of spontaneous pancreatitis and increases the severity of pancreatitis(Fig.3). With the expression of human PRSS1R122H from its native promoter, we created a human relevant animal model of spontaneous hereditary pancreatitis. This model is valuable to studying the underlying mechanisms of this disease and its progression to pancreatic cancer.
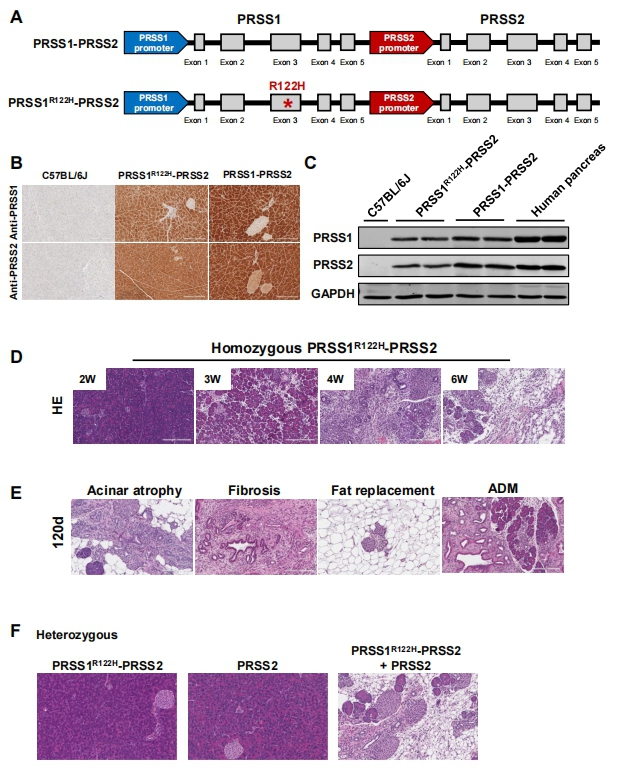
Fig.3 Transgenic wild-type human PRSS2 and PRSS1R122H expressions cooperatively initiated spontaneous pancreatitis
Reference
[1]Yang X, Yao L, Dai L, et al. Alcohol predisposes obese mice to acute pancreatitis via adipose triglyceride lipase-dependent visceral adipocyte lipolysis [J]. Gut. 2022, gutjnl-2022-326958. (IF=23.059)
[2]Choi J, Oh TG, Jung HW, et al. Estrogen-Related Receptor γ Maintains Pancreatic Acinar Cell Function and Identity by Regulating Cellular Metabolism [J]. Gastroenterology. 2022, 163(1):239-256. (IF=22.682)
[3]Wang J, Wan J, Wang L, et al. Wild-Type Human PRSS2 and PRSS1R122H Cooperatively Initiate Spontaneous Hereditary Pancreatitis in Transgenic Mice [J]. Gastroenterology. 2022, 163(1):313-315.e4. (IF=22.682)
Cloud-Clone not only provides a variety of endocrine system disease animal models, covering pancreatitis, thyroiditis, diabetes, high uric acid, non-alcoholic fatty liver, hyperlipidemia and other common diseases. We also have various endocrine system disease detection indicators and the above-mentioned ATGL, ERR and other related products, which can help the majority of scientific researchers to conduct research on endocrine system diseases.