New discovery of potential therapeutic targets for malaria
Malaria is a deadly infectious disease, caused by protist parasites of the genus Plasmodium, that affects 241 million people worldwide. Current antimalarial treatments are rapidly losing efficacy, and standard-of-care artemisinin combination therapies fail to cure infections in ~50% of patients in some regions of Asia. Clinically validated resistance to artemisinins has now been detected in Africa, where most malaria deaths occur. New treatments with novel modes of action are urgently needed to overcome existing resistance, expand possible treatment options, and enable more effective combination therapies.
1. Activation of cGAS-STING by Lethal Malaria N67C Dictates Immunity and Mortality through Induction of CD11b+Ly6Chi Proinflammatory Monocytes
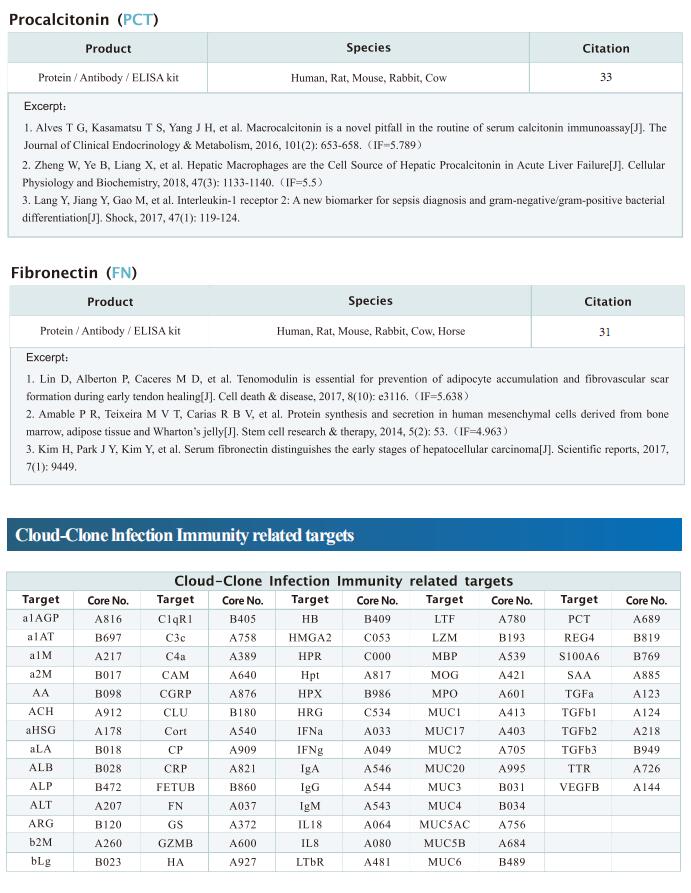
Cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING) play critical roles in the innate immunity against infectious diseases. Rong-Fu Wang, Department of Medicine, and Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, USA, and his team identified that cGAS-STING signaling plays a detrimental role in regulating anti-malaria immunity[1]. cGAS or STING deficiency in mice markedly prolongs mouse survival during lethal malaria Plasmodium yoelii nigeriensis N67C infections by reducing late interleukin (IL)-6 production. Mechanistically, cGAS/STING recruits myeloid differentiation factor 88 (MyD88) and specifically induces the p38-dependent signaling pathway for late IL-6 production, which, in turn, expands CD11b+Ly6Chi proinflammatory monocytes to inhibit immunity(Fig.1). Moreover, the blockage or ablation of the cGAS-STING-MyD88-p38-IL-6 signaling axis or the depletion of CD11b+Ly6Chi proinflammatory monocytes provides mice a significant survival benefit during N67C and other lethal malaria-strain infections. These findings provide clarity regarding the previously unrecognized role of the cGAS-STING-MyD88-p38 axis in modulating immunity against lethal malaria infections, which provides potential opportunities for exploring novel therapies.
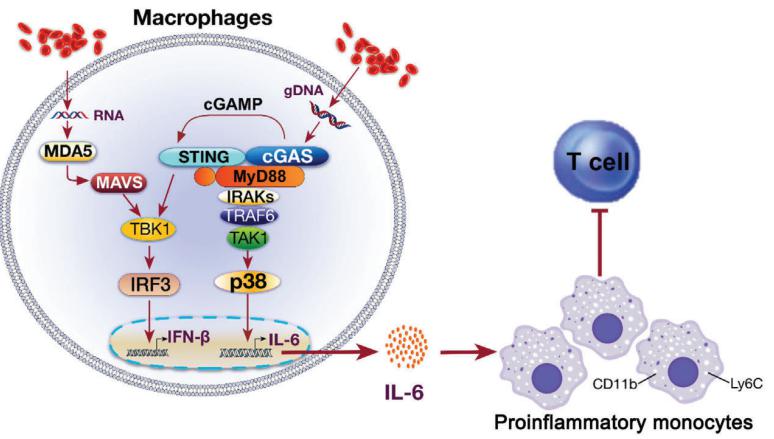
Fig.1 Activation of cGAS-STING by Lethal Malaria N67C Dictates Immunity and Mortality through Induction of CD11b+Ly6Chi Proinflammatory Monocytes
2. Functional genomics of RAP proteins and their role in mitoribosome regulation in Plasmodium falciparum
The RAP (RNA-binding domain abundant in Apicomplexans) protein family has been identified in various organisms. Karine G. Le Roch, Department of Molecular, Cell and Systems Biology, University of California Riverside, USA, and his team used inducible knockdown studies in the human malaria parasite, Plasmodium falciparum, to show that two RAP proteins, PF3D7_0105200 (PfRAP01) and PF3D7_1470600 (PfRAP21), are essential for parasite survival and localize to the mitochondrion[2]. Using transcriptomics, metabolomics, and proteomics profiling experiments, they further demonstrated that these RAP proteins are involved in mitochondrial RNA metabolism. Using high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (eCLIP-seq), they validated that PfRAP01 and PfRAP21 are true RNA-binding proteins and interact specifically with mitochondrial rRNAs. Finally, mitochondrial enrichment experiments followed by deep sequencing of small RNAs demonstrate that PfRAP21 controls mitochondrial rRNA expression(Fig.2). The results confirm an essential role of PfRAP01 and PfRAP21 proteins in regulation of the parasite’s mitoribosome, could open new opportunities for selectively targeting the Plasmodium proteins for therapeutic purposes.
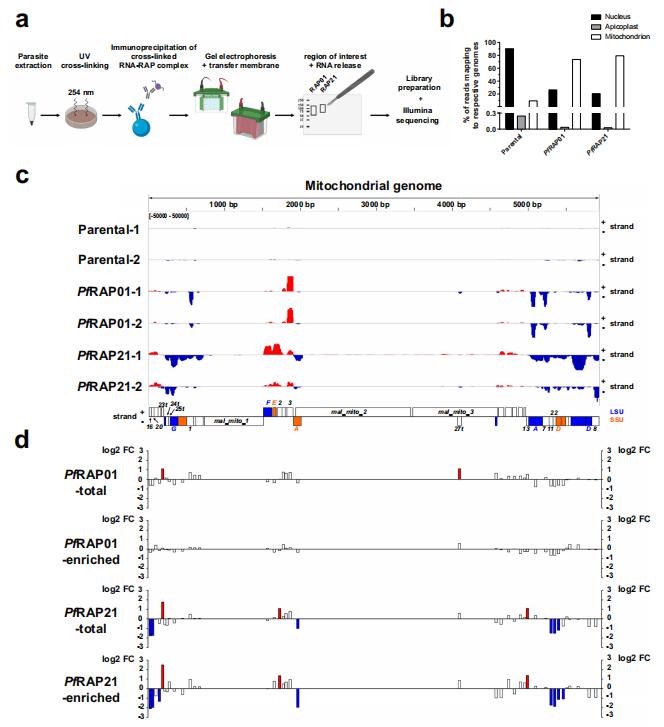
Fig.2 PfRAP01 and PfRAP21 participate in mitoribosome regulation
3. Reaction hijacking of tyrosine tRNA synthetase as a new whole-of-life-cycle antimalarial strategy
Aminoacyl transfer RNA (tRNA) synthetases (aaRSs) are attractive drug targets. Leann Tilley, Department of Biochemistry and Pharmacology, Bio21 Molecular Science and Biotechnology Institute, The University of Melbourne, Australia, and his team presented class I and II aaRSs as previously unrecognized targets for adenosine 5′-monophosphate–mimicking nucleoside sulfamates[3]. The target enzyme catalyzes the formation of an inhibitory amino acid–sulfamate conjugate through a reaction-hijacking mechanism. They identified adenosine 5′-sulfamate as a broad-specificity compound that hijacks a range of aaRSs and ML901 as a specific reagent that hijacks a single aaRS in the malaria parasite Plasmodium falciparum, namely tyrosine RS (PfYRS)(Fig.3). ML901 exerts whole-life-cycle–killing activity with low nanomolar potency and single-dose efficacy in a mouse model of malaria. Therefore, nucleoside sulfamate may be a new antimalarial strategy.
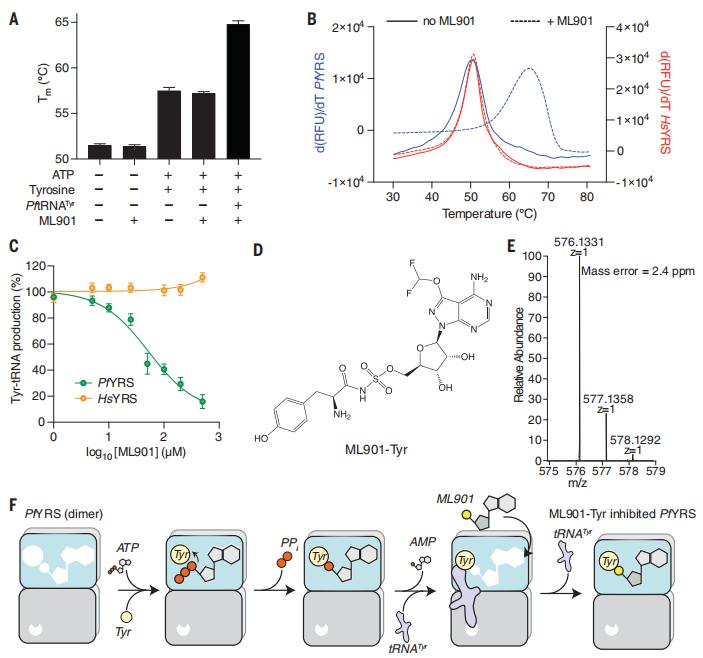
Fig.3 ML901 inhibits PfYRS by a reactionhijacking mechanism
References
[1]Du Y, Luo Y, Hu Z, et al. Activation of cGAS-STING by Lethal Malaria N67C Dictates Immunity and Mortality through Induction of CD11b+ Ly6Chi Proinflammatory Monocytes [J]. Adv Sci (Weinh). 2022, e2103701. (IF=17.521)
[2]Hollin T, Abel S, Falla A, et al. Functional genomics of RAP proteins and their role in mitoribosome regulation in Plasmodium falciparum [J]. Nat Commun. 2022, 13(1):1275. (IF=17.693)
[3]Xie SC, Metcalfe RD, Dunn E, et al. Reaction hijacking of tyrosine tRNA synthetase as a new whole-of-life-cycle antimalarial strategy [J]. Science. 2022, 376(6597):1074-1079. (IF=63.712)
Cloud-Clone can not only provide a variety of commonly used infection immune detection indicators related products, including PCT, CRP, IL-6, IL-10, etc., but also has the above-mentioned CGAS-STING signal, aaRS and other related products, which can help the majority of scientific researchers to conduct infectious disease research.