New discovery on the pathogenesis of kidney diseases
The kidneys, as a major filter organ for the blood and the key organ responsible for maintaining total body water balance and circulatory pressure, receive a rich blood supply by which they monitor and modify the functional status of multiple organ systems. Overall, 10% of the world's adults had some form of kidney disease. Kidney diseases are related to a massive financial burden.
New discovery on the pathogenesis of kidney diseases
Chronic kidney disease (CKD) is a clinical syndrome secondary to the definitive change in function and/or structure of the kidney and is characterized by its irreversibility and slow and progressive evolution. CKD affects a variety of metabolic pathways, resulting in changes in protein and energy homeostasis, abnormal protein catabolism, acid-base disorder and hormone dysfunction. In addition, CKD pathology is associated with a higher risk of complications and death. Understanding the molecular mechanisms that lead to CKD will help facilitate the development of new therapeutic strategies to prevent or mitigate disease progression.
1. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy
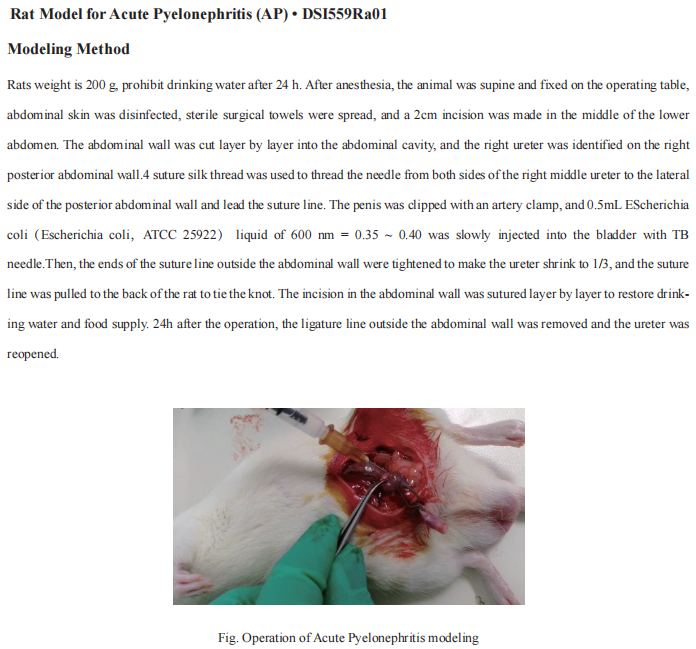
Ureteral obstruction is one of the most common problems in obstructive nephropathy. Renal tubular cell (RTC) death and inflammation contribute to the progression of obstructive nephropathy. Yanfang Xu, the First Affiliated Hospital, Fujian Medical University, and his team showed that Gasdermin E (GSDME) expression level and GSDME-N domain generation determined the RTC fate response to TNFα under the condition of oxygen-glucose-serum deprivation[1]. Deletion of Caspase-3 (Casp3) or Gsdme alleviated renal tubule damage and inflammation and finally prevented the development of hydronephrosis and kidney fibrosis after ureteral obstruction. They further showed that HMGB1 released from pyroptotic RTCs amplified inflammatory responses, which critically contributed to renal fibrogenesis. Specific deletion of Hmgb1 in RTCs alleviated caspase11 and IL-1β activation in macrophages. The results uncovered that TNFα/ Casp3/GSDME-mediated pyroptosis is responsible for the initiation of ureteral obstruction-induced renal tubule injury(Fig.1), which subsequentially contributes to the late-stage progression of hydronephrosis, inflammation, and fibrosis. This novel mechanism will provide valuable therapeutic insights for the treatment of obstructive nephropathy.
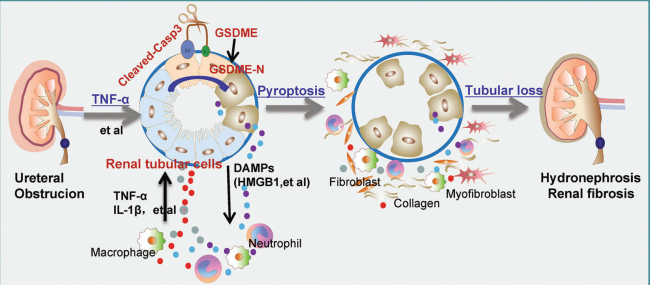
Fig.1 Caspase3/GSDME-mediated pyroptosis in the development of obstructive nephropathy
2. PP2Acα promotes macrophage accumulation and activation to exacerbate RTC death and kidney fibrosis
Macrophage accumulation and activation play an essential role in kidney fibrosis. Deciphering the mechanisms regulating macrophage activation and the interplay between macrophages and tubular cells is necessary for retarding kidney fibrosis. By analyzing the kidney tissues from patients and animal models with kidney fibrosis, Chunsun Dai, the 2nd Affiliated Hospital, Nanjing Medical University, and his team found a large induction of PP2Acα in macrophages [2]. They then generated a mouse model with inducible macrophage ablation of PP2Acα. The knockouts developed less renal fibrosis, macrophage accumulation, or tubular cell death after unilateral ureter obstruction or ischemic reperfusion injury compared to control littermates. Further analysis revealed that PP2Acα deficiency resulted in decreased cell motility by inhibiting Rap1 activity, and PP2Acα−/− macrophages resulted in less RTC death attributed to downregulated Stat6-mediated tumor necrosis factor α (TNFα) production in macrophages. This study demonstrates that PP2Acα promotes macrophage accumulation and activation, hence accelerates tubular cell death and kidney fibrosis through regulating Rap1 activation and TNFα production(Fig.2).
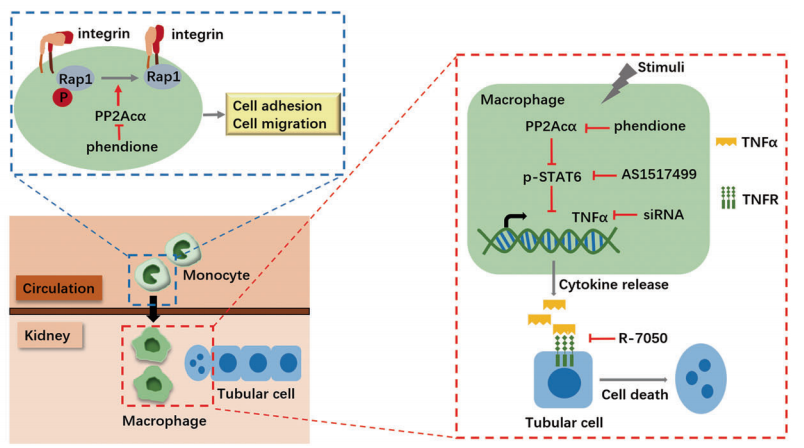
Fig.2 PP2Acα promotes macrophage accumulation and activation to exacerbate RTC death and kidney fibrosis through activating Rap1 and TNFα production
3. TRIM31 plays a critical role in hypertensive nephropathy by promoting proteasomal degradation of MAP3K7
Renal fibrosis and inflammation are critical for the initiation and progression of hypertensive renal disease (HRD). The tripartite motif (TRIM) family of proteins is an evolutionarily conserved E3 ubiquitin ligase family. Early studies indicated that TRIM31 plays a key role in a broad range of biological events, particularly, in the innate immune response, NLRP3 inflammasome activation, intestinal microbiota composition, and intestinal autophagy. Yun Zhang, Qilu Hospital, Cheeloo College of Medicine, and his team aimed to elucidate the role of TRIM31 in the pathogenesis of HRD [3]. They found that TRIM31 was markedly reduced in both human and mouse HRD renal tissues. A TRIM31-/- mice was thus constructed and showed significantly aggravated hypertension-induced renal dysfunction, fibrosis, and inflammation, following chronic AngII infusion compared with TRIM31+/+ mice. Overexpression of TRIM31 markedly ameliorated renal dysfunction, fibrotic and inflammatory response in AngII-induced HRD. Mechanistically, TRIM31 interacted with and catalyzed the K48-linked polyubiquitination of lysine 72 on Mitogen-activated protein kinase kinase kinase 7 (MAP3K7), followed by the proteasomal degradation of MAP3K7, which further negatively regulated TGF-β1-mediated Smad and MAPK/NF-κB signaling pathways, which slowed inflammation and renal fibrosis, ultimately inhibiting HRD progression(Fig.3).
Fig.3 reduction in TRIM31 leads to decreased ubiquitylation of MAP3K7, thereby activating the TGF-β1-MAP3K7-pSmad2/3 and TGF-β1-MAP3K7-NF-κB/MAPK pathways, which contributes to kidney injury associated with hypertension
References
[1]Li Y, Yuan Y, Huang ZX, et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy [J]. Cell Death Differ. 2021, 28(8):2333-2350. (IF=15.828)
[2]Liang Y, Sun X, Wang M, et al. PP2Acα promotes macrophage accumulation and activation to exacerbate tubular cell death and kidney fibrosis through activating Rap1 and TNFα production [J]. Cell Death Differ. 2021, 28(9):2728-2744. (IF=15.828)
[3]Zhang J, Cao L, Wang X, et al. The E3 ubiquitin ligase TRIM31 plays a critical role in hypertensive nephropathy by promoting proteasomal degradation of MAP3K7 in the TGF-β1 signaling pathway [J]. Cell Death Differ. 2021, 10.1038/s41418-021-00874-0. (IF=15.828)
Cloud-Clone can not only provide animal models of various urinary system diseases, including renal fibrosis, pyelonephritis, renal failure, glomerulonephritis, kidney stones, hydronephrosis and other common urinary system diseases. It also has various renal disease detection indicators and the above-mentioned HMGB1, Caspase-3, IL-1β, Stat6, TNFα protein detection products, which can help the general scientific research workers to carry out renal disease related research.