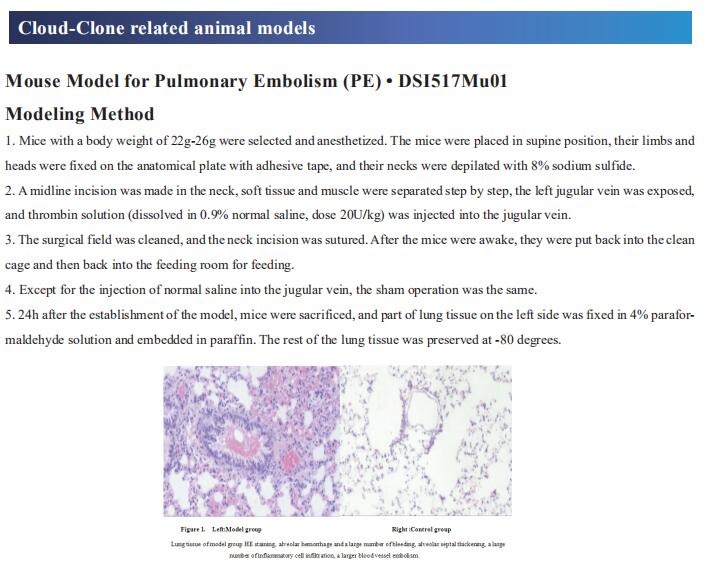
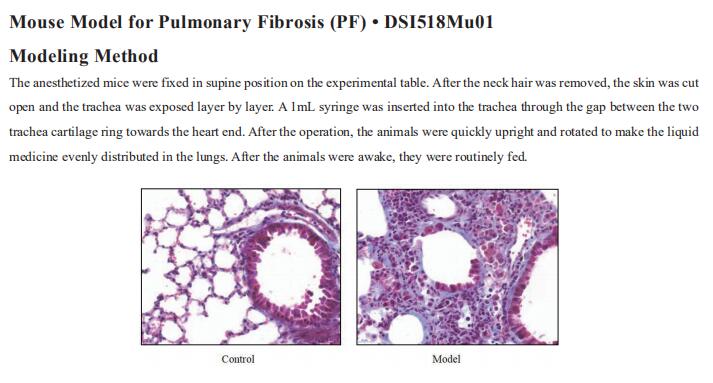
New findings in cystic fibrosis research
Cystic fibrosis (CF) is a multisystem autosomal recessive disease that is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR protein is an ion channel that functions to regulate the transepithelial movement of anions predominantly in mucus-secreting tissues, thereby acting to orchestrate the balancing of salt and fluid levels in mucus secretions. Disease-causing mutations in this gene affect multiple organs in CF including the gut, pancreas, and the liver, but it is the impact of dysregulated CFTR function in the airways that proves particularly harmful to individuals with CF. Dehydration of the airway surface liquid and thickening of mucus secretions prevents effective airway mucociliary clearance and provides fertile ground for harmful bacteria, leading to recurring bouts of infection throughout life. Recently, multiple papers have reported on CF related studies, which may help develop more effective CF treatment methods to improve patient prognosis.
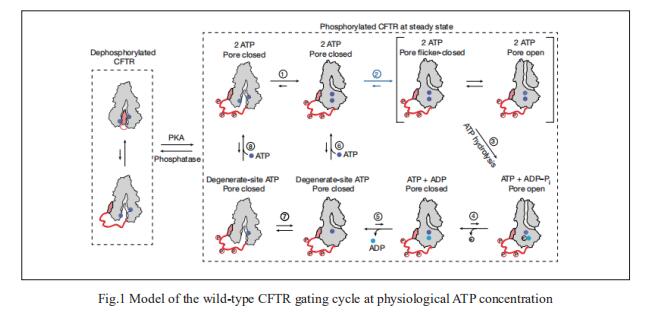
1. CFTR function, pathology and pharmacology at single-molecule resolution
Electrophysiological properties of CFTR have been analysed for decades. However, direct correlations between the essential functions of CFTR and extant structures are lacking at present. Jue Chen, Laboratory of Membrane Biology and Biophysics, The Rockefeller University, USA, and his team combined ensemble functional measurements, singlemolecule fuorescence resonance energy transfer, electrophysiology and kinetic simulations to show that the two nucleotide-binding domains (NBDs) of human CFTR dimerize before channel opening[1]. CFTR exhibits an allosteric gating mechanism in which conformational changes within the NBD-dimerized channel, governed by ATP hydrolysis, regulate chloride conductance (Fig.1). The potentiators ivacaftor and GLPG1837 enhance channel activity by increasing pore opening while NBDs are dimerized. Disease-causing substitutions proximal (G551D) or distal (L927P) to the ATPase site both reduce the efficiency of NBD dimerization. These findings collectively enable the framing of a gating mechanism that informs on the search for more efficacious clinical therapies.
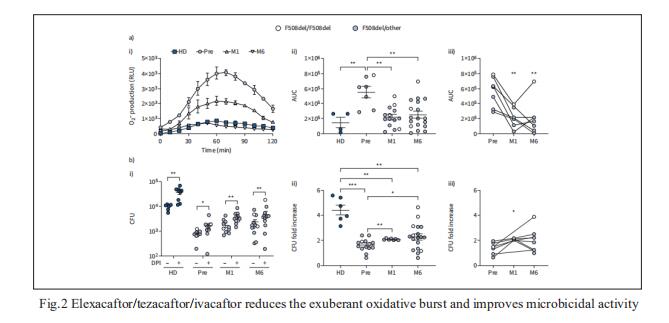
2. Elexacaftor/tezacaftor/ivacaftor corrects monocyte microbicidal deficiency in CF
In CF, monocytes and monocyte-derived macrophages have been shown to display defective phagocytosis and antimicrobial activity against relevant lung pathogens, including Pseudomonas aeruginosa. Thus, Fiorentina Ascenzioni, Department of Biology and Biotechnology “Charles Darwin”, Sapienza University of Rome, Italy, and his team addressed the effect of CFTR triple modulator therapy (elexacaftor/tezacaftor/ivacaftor (ETI)) on the activity of CF monocytes against P. aeruginosa[2]. Longitudinal analysis of the clinical parameters confirmed an improvement of lung function and lung microbiology by ETI. Both the phagocytic and microbicidal deficiencies of CF monocytes also improved significantly. Furthermore, they measured an exuberant oxidative burst in CF monocytes before therapy, which was reduced considerably by ETI (Fig.2). This led to an improvement of reactive oxygen species-dependent bactericidal activity. Inflammatory response to bacterial stimuli was also lowered compared with pre-therapy. CF on ETI therapy, in a real-life setting, in addition to clinical recovery, showed significant improvement in monocyte activity against P. aeruginosa, which may have contributed to the overall effect of ETI on pulmonary disease. This also suggests that CF monocyte dysfunctions may be specifically targeted to ameliorate lung function in CF.
3. Antigen specificity and cross-reactivity drive functionally diverse anti–Aspergillus fumigatus T cell responses in cystic fibrosis
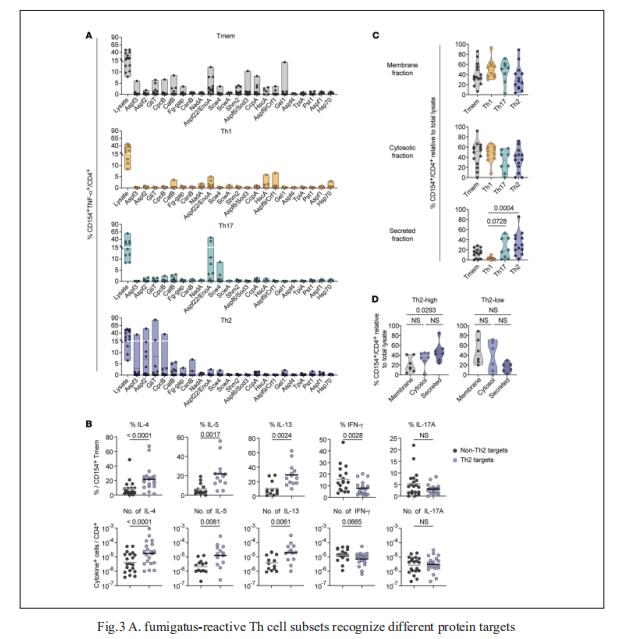
The fungus Aspergillus fumigatus causes a variety of clinical phenotypes in patients with CF. Th cells orchestrate immune responses against fungi, but the types of A. fumigatus–specific Th cells in CF and their contribution to protective immunity or inflammation remain poorly characterized. Petra Bacher, Institute of Clinical Molecular Biology, Christian-Albrecht University of Kiel, Germany, and his team used antigen-reactive T cell enrichment (ARTE) to investigate fungus-reactive Th cells in peripheral blood of pwCF and healthy controls[3]. They showed that clonally expanded, high-avidity A. fumigatus-specific effector Th cells, which were absent in healthy donors, developed in CF. Individual patients were characterized by distinct Th1-, Th2-, or Th17-dominated responses that remained stable over several years. These different Th subsets target different A. fumigatus proteins (Fig.3), indicating that differential antigen uptake and presentation directs Th cell subset development. The dissection of heterogenous Th cell reactions and their target antigens in CF can facilitate the development of targeted therapeutic strategies.
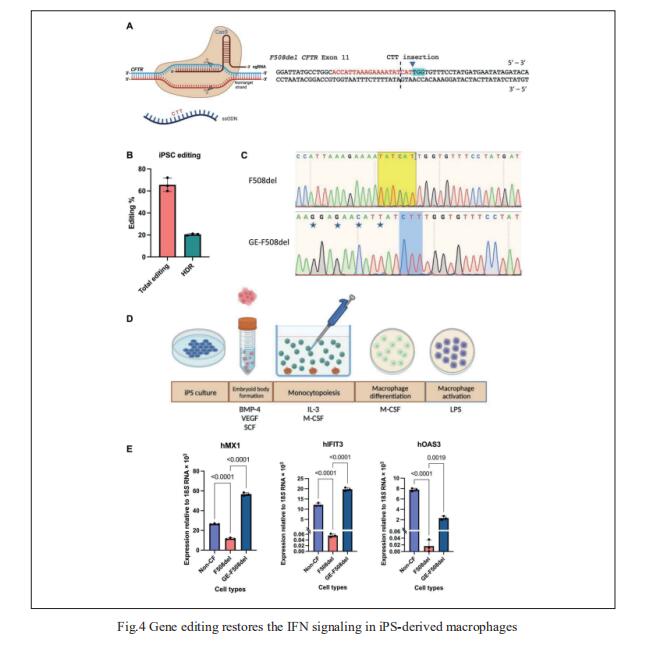
4. CAGE sequencing reveals CFTR-dependent dysregulation of type I IFN signaling in activated CF macrophages
Dysregulation of macrophage immune function may be a key facet governing the progression of CF lung disease, but the underlying mechanisms are not fully understood. Robert D. Gray, University of Edinburgh Centre for Inflammation Research, Queen’s Medical Research Institute, UK, and his team used 5′ end centered transcriptome sequencing to profile P. aeruginosa LPS-activated human CF macrophages, showing that CF and non-CF macrophages deploy substantially distinct transcriptional programs at baseline and following activation[4]. This includes a significantly blunted type I IFN signaling response in activated patient cells relative to healthy controls that was reversible upon in vitro treatment with CFTR modulators in patient cells and by CRISPR-Cas9 gene editing to correct the F508del mutation in patient-derived iPSC macrophages (Fig.4). These findings illustrate a previously unidentified immune defect in human CF macrophages that is CFTR dependent and reversible with CFTR modulators, thus providing new avenues in the search for effective anti-inflammatory interventions in CF.
References
[1]Levring J, Terry DS, Kilic Z, Fitzgerald G, Blanchard SC, Chen J. CFTR function, pathology and pharmacology at single-molecule resolution [J]. Nature. 2023,616(7957):606-614. (IF=69.504)
[2]Cavinato L, Luly FR, Pastore V, et al. Elexacaftor/tezacaftor/ivacaftor corrects monocyte microbicidal deficiency in cystic fibrosis [J]. Eur Respir J. 2023,61(4):2200725. (IF=33.795)
[3]Schwarz C, Eschenhagen P, Schmidt H, et al. Antigen specificity and cross-reactivity drive functionally diverse anti-Aspergillus fumigatus T cell responses in cystic fibrosis [J]. J Clin Invest. 2023,133(5):e161593. (IF=19.456)
[4]Gillan JL, Chokshi M, Hardisty GR, et al. CAGE sequencing reveals CFTR-dependent dysregulation of type I IFN signaling in activated cystic fibrosis macrophages [J]. Sci Adv. 2023,9(21):eadg5128. (IF=14.957)
Cloud-Clone can not only provide animal models of a variety of respiratory diseases, including chronic obstructive pulmonary disease, asthma, bronchitis, pulmonary embolism, pneumonia, pulmonary fibrosis, etc., covering common respiratory diseases. There are also various respiratory disease detection indicators and the above CFTR, IFN and other protein-related products, which can help the majority of scientific researchers to carry out respiratory disease related research.