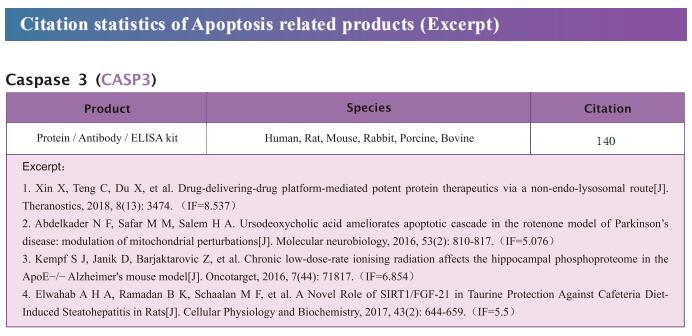
New findings on the mechanism of pyroptosis
As a lytic and inflammatory type of regulated cell death, pyroptosis is characterized by cell swelling, lysis, and the release of many proinflammatory factors, including IL1β, IL-18, ATP, and HMGB1. Dying cells activate pyroptosis through the following two main approaches: (i) GSDMD (gasdermin D)-dependent activation regulated by caspase 1/4/5/11 and (ii) GSDME-dependent activation regulated by caspase 3. Activated caspases cleave the hinge region between the N- and C-terminal domains of GSDMD or GSDME, releasing the segment with lethal activity and leading to pyroptosis. Few studies investigated the roles of other GSDM family members in pyroptosis, but GSDMA, GSDMB, and GSDMC also harbor a pore-formation domain and can induce pyroptosis. Recently, multiple papers have reported the studies related to pyroptosis, providing assistance for further understanding the mechanism of pyroptosis.
1. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin
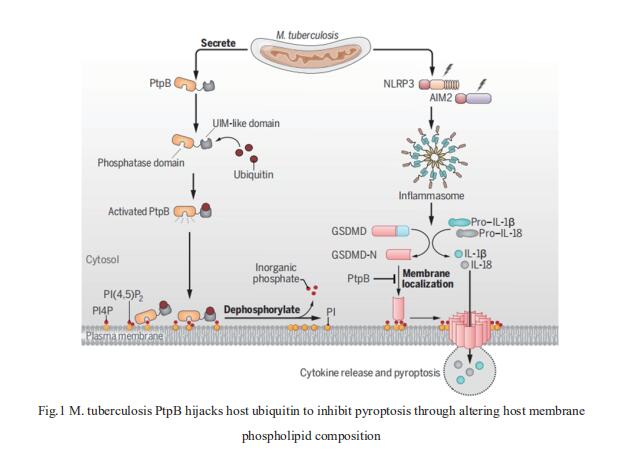
The inflammasome-mediated cleavage of gasdermin D (GSDMD) causes pyroptosis and inflammatory cytokine release to control pathogen infection, but how pathogens evade this immune response remains largely unexplored. Cui Hua Liu, CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, China, and his team identified the known protein phosphatase PtpB from Mycobacterium tuberculosis as a phospholipid phosphatase inhibiting the host inflammasome-pyroptosis pathway[1]. Mechanistically, PtpB dephosphorylated phosphatidylinositol-4-monophosphate and phosphatidylinositol-(4,5)-bisphosphate in host cell membrane, thus disrupting the membrane localization of the cleaved GSDMD to inhibit cytokine release and pyroptosis of macrophages (Fig.1). Notably, this phosphatase activity requires PtpB binding to ubiquitin. Disrupting phospholipid phosphatase activity or the ubiquitin-interacting motif of PtpB enhanced host GSDMD-dependent immune responses and reduced intracellular pathogen survival. Thus, pathogens inhibit pyroptosis and counteract host immunity by altering host membrane composition.
2. Bacterial gasdermins reveal an ancient mechanism of cell death
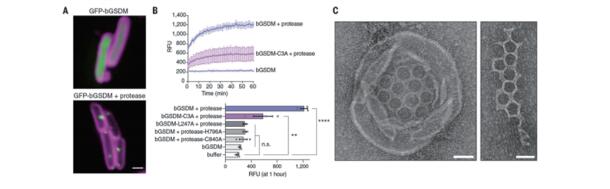
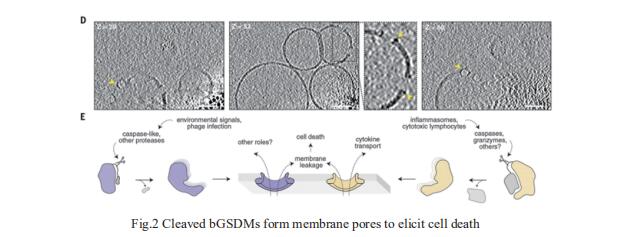
Gasdermin proteins form large membrane pores in human cells that release immune cytokines and induce lytic cell death. Gasdermin pore formation is triggered by caspase-mediated cleavage during inflammasome signaling and is critical for defense against pathogens and cancer. Philip J. Kranzusch, Department of Microbiology, Harvard Medical School, USA, and his team discovered gasdermin homologs encoded in bacteria that defended against phages and executed cell death[2]. Structures of bacterial gasdermins revealed a conserved pore-forming domain that was stabilized in the inactive state with a buried lipid modification. Bacterial gasdermins were activated by dedicated caspase-like proteases that catalyzed site-specific cleavage and the removal of an inhibitory C-terminal peptide. Release of autoinhibition induced the assembly of large and heterogeneous pores that disrupted membrane integrity (Fig.2). Thus, pyroptosis is an ancient form of regulated cell death shared between bacteria and animals.
3. Structural mechanisms for regulation of GSDMB pore-forming activity
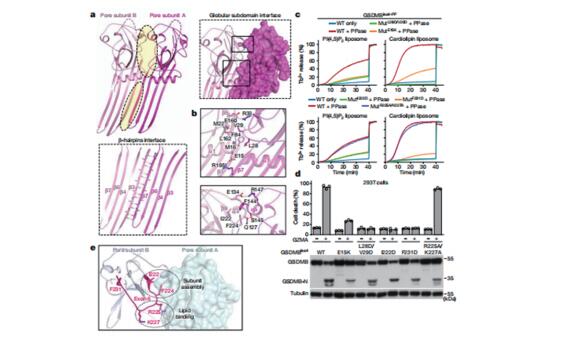
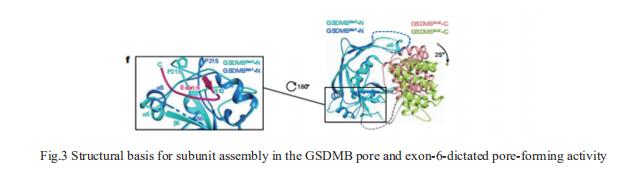
Cytotoxic lymphocyte-derived granzyme A (GZMA) cleaves GSDMB, a gasderminfamily pore-forming protein, to trigger target cell pyroptosis. GSDMB and the charter gasdermin family member GSDMD have been inconsistently reported to be degraded by the Shigella fexneri ubiquitin-ligase virulence factor IpaH7.8. Whether and how IpaH7.8 targets both gasdermins is undefned, and the pyroptosis function of GSDMB has even been questioned recently. Jingjin Ding, National Institute of Biological Sciences, China, and his team reported the crystal structure of the IpaH7.8–GSDMB complex, which shows how IpaH7.8 recognizes the GSDMB pore-forming domain[3]. The structure of full-length GSDMB suggests stronger autoinhibition than in other gasdermins. GSDMB has multiple splicing isoforms that are equally targeted by IpaH7.8 but exhibit contrasting pyroptotic activities. Presence of exon 6 in the isoforms dictates the pore-forming, pyroptotic activity in GSDMB (Fig.3). They determined the cryo-electron microscopy structure of the 27-fold-symmetric GSDMB pore and depict conformational changes that drive pore formation. The structure uncovers an essential role for exon-6-derived elements in pore assembly, explaining pyroptosis defciency in the non-canonical splicing isoform used in recent studies. Diferent cancer cell lines have markedly diferent isoform compositions, correlating with the onset and extent of pyroptosis following GZMA stimulation. The study illustrated fine regulation of GSDMB pore-forming activity by pathogenic bacteria and mRNA splicing and defnes the underlying structural mechanisms.
References
[1]Chai Q, Yu S, Zhong Y, et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin [J]. Science. 2022;378(6616):eabq0132. (IF=63.714)
[2]Johnson AG, Wein T, Mayer ML, et al. Bacterial gasdermins reveal an ancient mechanism of cell death [J]. Science. 2022;375(6577):221-225. (IF=63.714)
[3]Zhong X, Zeng H, Zhou Z, et al. Structural mechanisms for regulation of GSDMB pore-forming activity [J]. Nature. 2023;10.1038/s41586-023-05872-5. (IF=69.504)
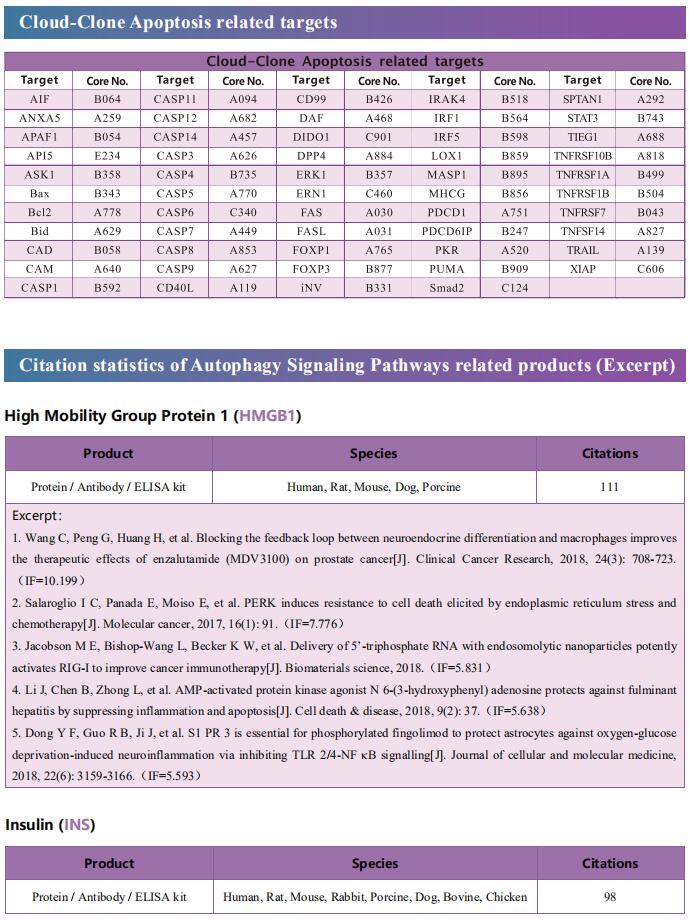
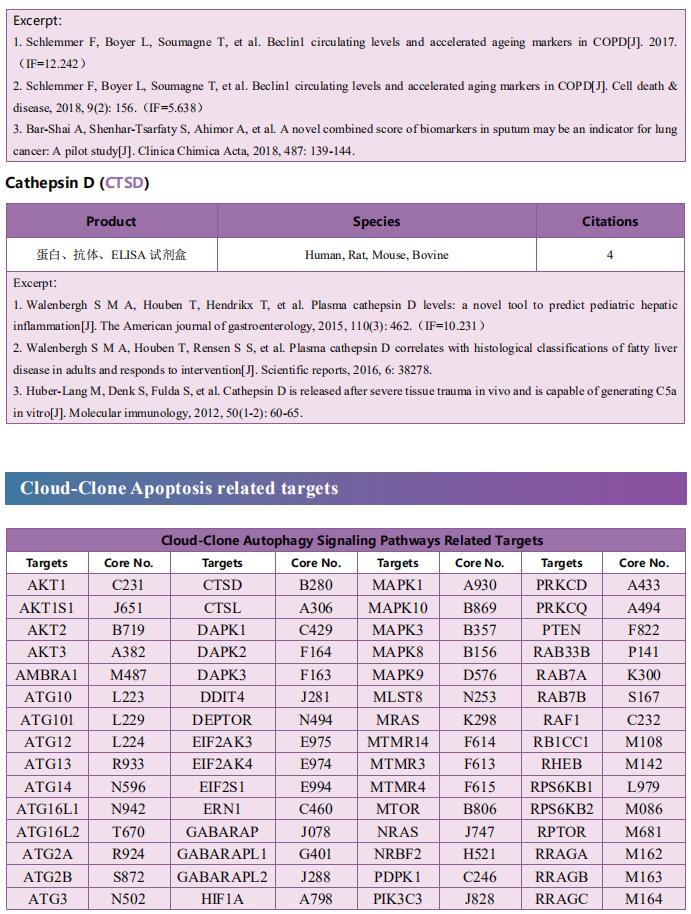
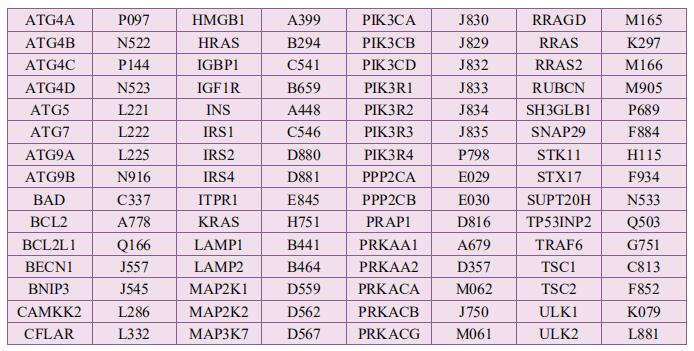
Cloud-Clone can not only provide a variety of cell death (apoptosis, autophagy, ferroptosis, pyroptosis) signaling pathway related products, including TNFα, CASP3, CTSK, NFkB, CASP9, Bcl2, BECN1, CTSD, CTSL, ERK1, ERK2, etc. In addition, we also have the above-mentioned GSDMD, GSDMA, GSDMC, GZMA and other related protein detection products, which can help the majority of scientific researchers to conduct cell death related research.