New findings on the pathogenesis of psoriasis
Psoriasis is a common chronic skin disease and affects 2-3% of the global population. It is characterized by well-demarcated, erythematous plaques covered by silvery-white scales, typically occurring in a symmetric distribution involving the elbows, knees, trunk and scalp. Individuals with psoriasis are at an increased risk of developing other chronic and serious health diseases. These comorbid diseases include psoriatic arthritis, metabolic syndrome or components of the syndrome, cardiovascular disorders, and several other diseases such as anxiety and depression, non-alcoholic fatty liver disease, Crohn’s disease, and lymphoma. At present, the treatment options for psoriasis include topical corticosteroids, vitamin D analogues, calcineurin inhibitors, keratolytics, targeted phototherapy, and biological agents. Although systemic therapy of psoriasis has advanced considerably there is still an unmet need for more effective topical therapies and a more widespread use of biosimilars. Recently, a number of papers have reported the pathogenesis of psoriasis, which may provide help to further improve the prognosis of patients.
1. FXYD3 enhances IL-17A signaling to promote psoriasis by competitively binding TRAF3 in keratinocytes
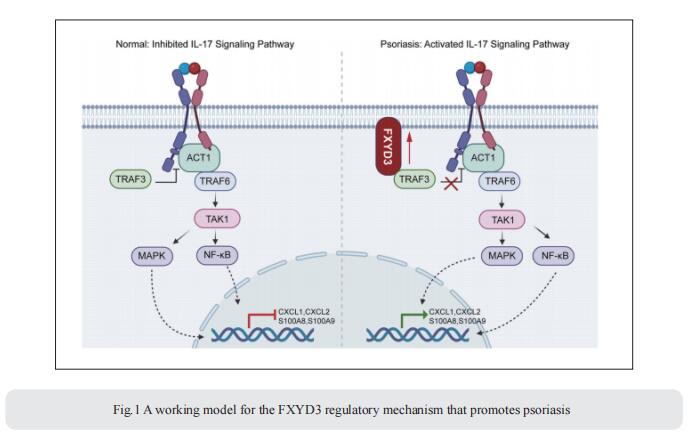
FXYD3, also known as Mat-8, belongs to the FXYD protein family. FXYD3 is mainly expressed in the skin as well as in the colon, stomach, and uterus. However, the pathological roles and mechanisms of FXYD3 in skin diseases such as psoriasis remain largely unknown. Qingqing Wang, Institute of Immunology, Zhejiang University School of Medicine, China, and his team showed that the expression of FXYD3 is significantly increased in the lesional skin of psoriasis patients and mice with imiquimod (IMQ)-induced psoriasis[1]. IL-17A, a cytokine important for the development of psoriatic lesions, contributes to FXYD3 expression in human primary keratinocytes. FXYD3 deletion in keratinocytes attenuated the psoriasis-like phenotype and inflammation in an IMQ-induced psoriasis model. Importantly, FXYD3 promotes the formation of the IL-17R-ACT1 complex by competing with IL-17R for binding to TRAF3 and then enhances IL-17A signaling in keratinocytes (Fig.1). This promotes the activation of the NF-κB and MAPK signaling pathways and leads to the expression of proinflammatory factors. These results clarify the mechanism by which FXYD3 serves as a mediator of IL-17A signaling in keratinocytes to form a positive regulatory loop to promote psoriasis exacerbation. Targeting FXYD3 may serve as a potential therapeutic approach in the treatment of psoriasis.
2. Intestinal dysbiosis exacerbates the pathogenesis of psoriasis-like phenotype through changes in fatty acid metabolism
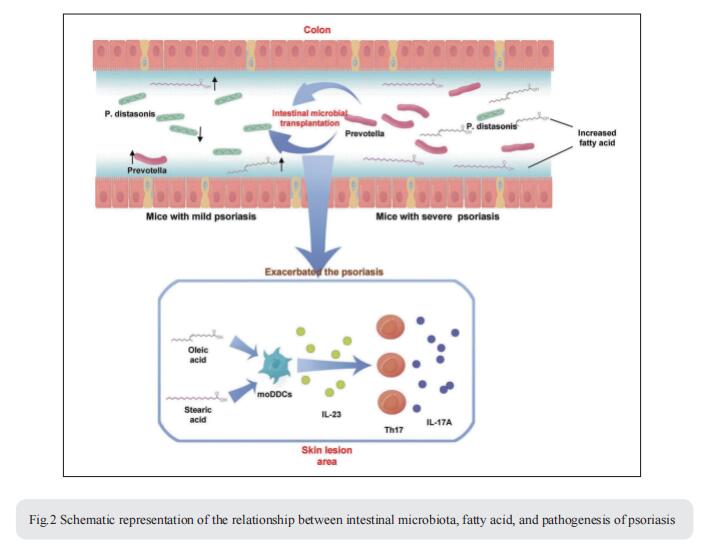
The intestinal microbiota has been associated with host immunity as well as psoriasis; however, the mechanism of intestinal microbiota regulating psoriasis needs to be demonstrated systematically. Jiong Li, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, West China Medical School, Sichuan University, and Collaborative Innovation Center for Biotherapy, China, and his team found that the severity of psoriasis-like skin phenotype was accompanied by changes in the composition of the intestinal microbiota[2]. They performed co-housing and fecal microbial transplantation (FMT) experiments using the K14-VEGF transgenic mouse model of psoriasis and demonstrated that the transfer of intestinal microbiota from mice with severe psoriasis-like skin phenotype exacerbated psoriasiform skin inflammation in mice with mild symptoms, including increasing the infiltration and differentiation of Th17, and increased the abundance of Prevotella, while decreasing that of Parabacteroides distasonis, in the colon. These alterations affected fatty acid metabolism, increasing the abundance of oleic and stearic acids. Meanwhile, gentamicin treatment significantly reduced the abundance of Prevotella and alleviated the psoriasis-like symptoms in both K14-VEGF mice and IMQ-induced psoriasis-like mice. Indeed, administration of oleic and stearic acids exacerbated psoriasis-like symptoms and increased Th17 and monocyte-derived dendritic cell infiltration in the skin lesion areas in vivo, as well as increased the secretion of IL-23 by stimulating DCs in vitro (Fig.2). At last, they found that, treatment of PDE-4 inhibitor alleviated psoriasis-like phenotype of K14-VEGF mice accompanied by the recovery of intestinal microbiota, including the decrease of Prevotella and increase of Parabacteroides distasonis. Overall, these findings reveal that the intestinal microbiota modulates host metabolism and psoriasis-like skin inflammation in mice, suggesting a new target for the clinical diagnosis and treatment of psoriasis.
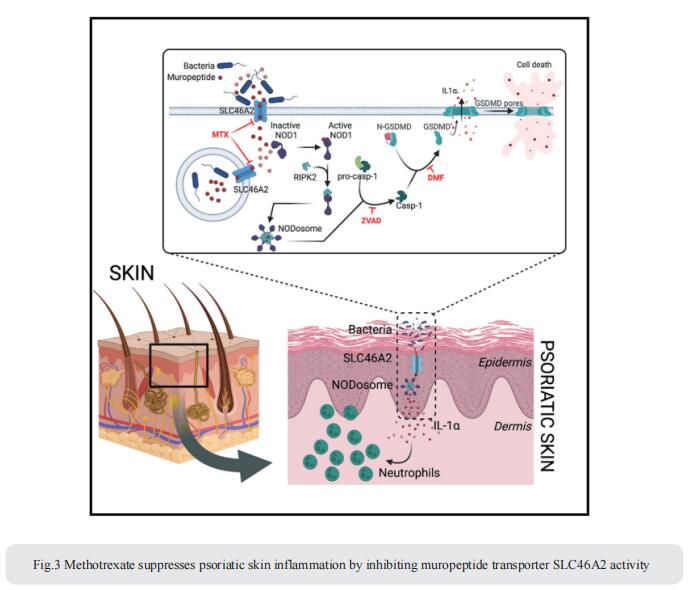
3. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity
Cytosolic innate immune sensing is critical for protecting barrier tissues. NOD1 and NOD2 are cytosolic sensors of small peptidoglycan fragments (muropeptides) derived from the bacterial cell wall. These muropeptides enter cells, especially epithelial cells, through unclear mechanisms. Neal Silverman, Program in Innate Immunity and Division of Infectious Diseases and Immunology, Department of Medicine, University of Massachusetts Chan Medical School, USA, focused on Slc46a2, which was highly expressed in mammalian epidermal keratinocytes, and showed that it was critical for the delivery of diaminopimelic acid (DAP)-muropeptides and activation of NOD1 in keratinocytes, whereas the related transporter Slc46a3 was critical for delivering the NOD2 ligand MDP to keratinocytes[3]. In a mouse model, Slc46a2 and Nod1 deficiency strongly suppressed psoriatic inflammation, whereas methotrexate, a commonly used psoriasis therapeutic, inhibited Slc46a2-dependent transport of DAP-muropeptides(Fig.3). Collectively, these studies define SLC46A2 as a transporter of NOD1-activating muropeptides, with critical roles in the skin barrier, and identify this transporter as an important target for anti-inflammatory intervention.
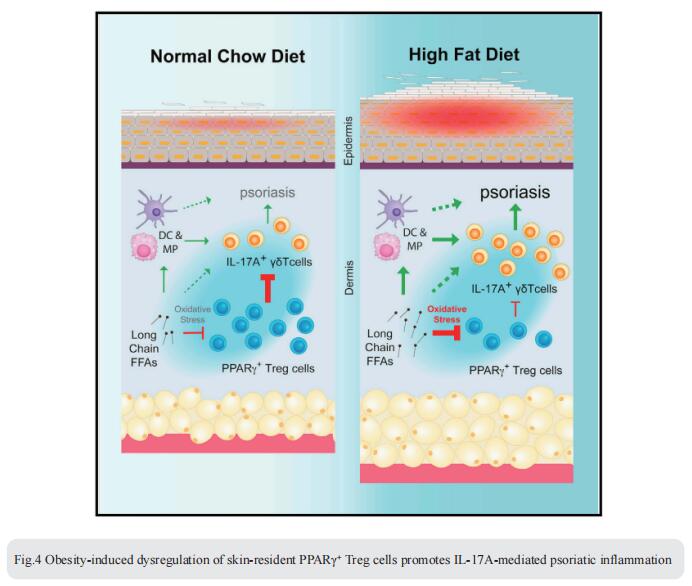
4. Obesity-induced dysregulation of skin-resident PPARγ+ Treg cells promotes IL-17A-mediated psoriatic inflammation
Obesity is a major risk factor for psoriasis, but how obesity disrupts the regulatory mechanisms that keep skin inflammation in check is unclear. Chaoran Li, Department of Microbiology and Immunology, Emory University School of Medicine, USA, and his team found that skin was enriched with a unique population of CD4+ Foxp3+ regulatory T (Treg) cells expressing the nuclear receptor peroxisome proliferation-activated receptor gamma (PPARγ)[4]. PPARγ drove a distinctive transcriptional program and functional suppression of IL-17A+ γδ T cell-mediated psoriatic inflammation. Diet-induced obesity, however, resulted in a reduction of PPARγ+ skin Treg cells and a corresponding loss of control over IL-17A+ γδ T cell-mediated inflammation. Mechanistically, PPARγ+ skin Treg cells preferentially took up elevated levels of long-chain free fatty acids in obese mice, which led to cellular lipotoxicity, oxidative stress, and mitochondrial dysfunction (Fig.4). Harnessing the anti-inflammatory properties of these PPARγ+ skin Treg cells could have therapeutic potential for obesityassociated inflammatory skin diseases.
References
[1]Yang W, He R, Qu H, et al. FXYD3 enhances IL-17A signaling to promote psoriasis by competitively binding TRAF3 in keratinocytes [J]. Cell Mol Immunol. 2023;20(3):292-304. (IF=24.1)
[2]Zhao Q, Yu J, Zhou H, et al. Intestinal dysbiosis exacerbates the pathogenesis of psoriasis-like phenotype through changes in fatty acid metabolism [J]. Signal Transduct Target Ther. 2023;8(1):40. (IF=39.3)
[3]Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity [J]. Immunity. 2023;56(5):998-1012.e8. (IF=32.4)
[4]Sivasami P, Elkins C, Diaz-Saldana PP, et al. Obesity-induced dysregulation of skin-resident PPARγ+ Treg cells promotes IL-17A-mediated psoriatic inflammation [J]. Immunity. 2023;56(8):1844-1861.e6. (IF=32.4)
Cloud-Clone can not only provide animal models of various autoimmune diseases, including psoriasis, myasthenia gravis, allergic encephalomyelitis, Sjogren's syndrome, systemic lupus erythematosus, rheumatoid arthritis, etc., covering common autoimmune diseases. We also have various autoimmune disease detection indicators and related products such as IL-17A, TRAF3, NOD1, NOD2, PPARγ, which can help the majority of scientific researchers to carry out autoimmune disease research.