New progress in antibody therapy of cancer
Cancer therapy using monoclonal antibodies (mAbs) is a rapidly developing field, due in large part to naturally favorable attributes such as specificity, potency, and metabolic stability. Most of them act by binding to transmembrane receptors or soluble ligands, thereby interfering with their signal transduction pathways, resulting in inhibition of tumor cell proliferation or angiogenesis. Due to the presence of an intact Fc domain, mAbs can evoke antibody-dependent cell-mediated cytotoxicity by attracting complement or effector cells of the human immune system to the cancer site. mAbs have also been used as targeting moieties for the delivery of nanomedicines or conjugating to cytotoxic drugs. Recently, a number of papers have reported the research on cancer antibody therapy, providing help for the treatment of cancer.
1. Ginseng polysaccharides potentiate the antitumour effect of anti-PD-1/PD-L1 immunotherapy
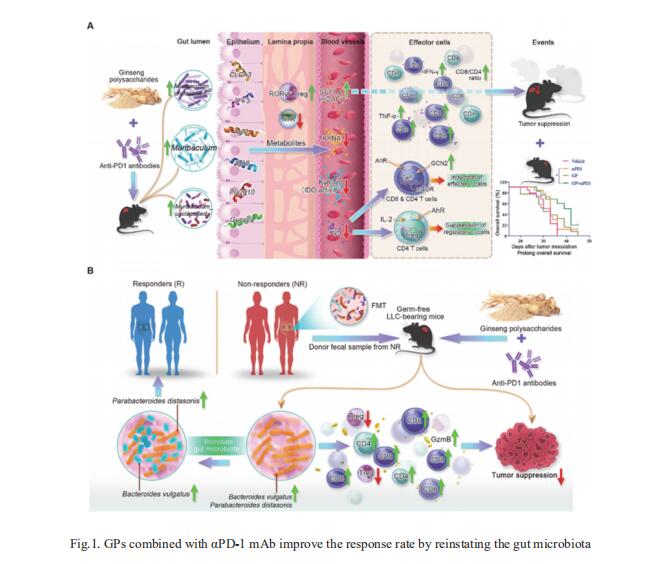
Programmed death 1 and its ligand 1 (PD-1/PD-L1) immunotherapy is promising for late-stage lung cancer treatment, however, the response rate needs to be improved. Gut microbiota plays a crucial role in immunotherapy sensitisation and Panax ginseng has been shown to possess immunomodulatory potential. Elaine Lai-Han Leung, State Key laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, China, and his team aimed to investigate whether the combination treatment of ginseng polysaccharides (GPs) and αPD-1 monoclonal antibody (mAb) could sensitise the response by modulating gut microbiota[1]. They found GPs increased the antitumour response to αPD-1 mAb by increasing the microbial metabolites valeric acid and decreasing L-kynurenine, as well as the ratio of Kyn/Trp, which contributed to the suppression of regulatory T cells and induction of Teffcells after combination treatment. Furthermore, the combination therapy sensitised the response to PD-1 inhibitor in the mice receiving microbes by faecal microbiota transplantation from six non-responders by reshaping the gut microbiota from non-responders towards that of responders (Fig.1). These results demonstrate that GPs combined with αPD-1 mAb may be a new strategy to sensitise non-small cell lung cancer patients to anti-PD-1 immunotherapy.
2. Reducing affinity as a strategy to boost immunomodulatory antibody agonism
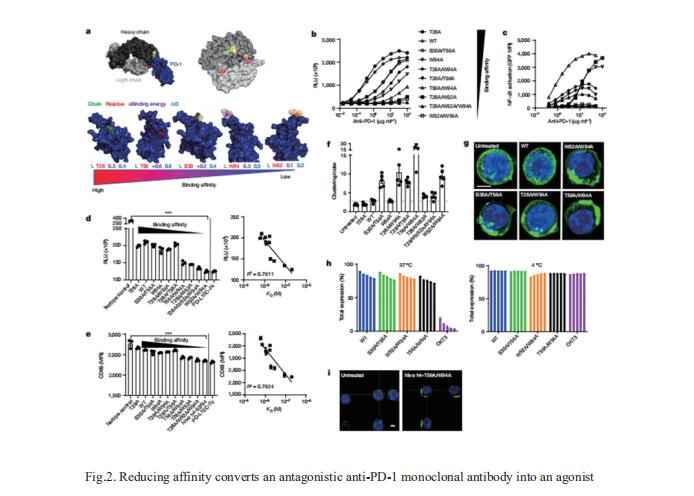
High affinity is routinely the goal for therapeutic antibody generation. However, in contrast to naturally occurring or direct-targeting therapeutic antibodies, immunomodulatory antibodies, which are designed to modulate receptor signalling, have not been widely examined for their affinity–function relationship. Mark S. Cragg, Antibody and Vaccine Group, Centre for Cancer Immunology, School of Cancer Sciences, University of Southampton Faculty of Medicine, UK, and his team examined three separate immunologically important receptors spanning two receptor superfamilies: CD40, 4-1BB and PD-1[2]. They showed that low rather than high affinity delivers greater activity through increased clustering. This approach delivered higher immune cell activation, in vivo Tcell expansion and antitumour activity in the case of CD40. Moreover, an inert anti-4-1BB monoclonal antibody was transformed into an agonist. Low-affinity variants of the clinically important antagonistic anti-PD-1 monoclonal antibody nivolumab also mediated more potent signalling and affected Tcell activation (Fig.2). These findings reveal a new paradigm for augmenting agonism across diverse receptor families and shed light on the mechanism of antibody-mediated receptor signalling.
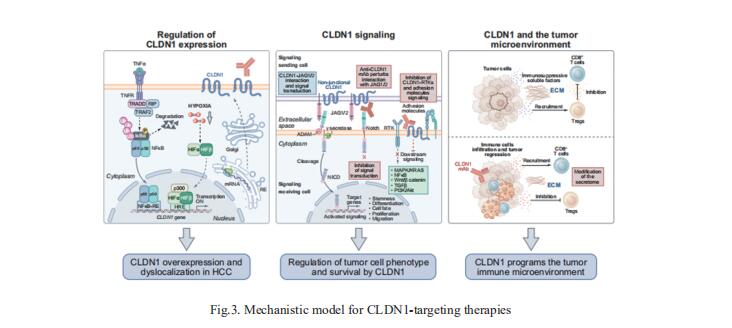
3. Treatment of HCC with claudin-1-specific antibodies suppresses carcinogenic signaling and reprograms the tumor microenvironment
Claudin-1 (CLDN1) is a membrane protein that is expressed at tight junctions, but it can also be exposed non-junctionally, such as on the basolateral membrane of the human hepatocyte. While CLDN1 within tight junctions is well characterized, the role of non-junctional CLDN1 and its role as a therapeutic target in hepatocellular carcinoma (HCC) remains unexplored. Thomas F. Baumert, Institut de Recherche sur les Maladies Virales et Hépatiques, France, and his team found that targeting non-junctional CLDN1 markedly suppressed tumor growth and invasion in cell line-based models of HCC and patient-derived 3D ex vivo models[3]. Moreover, the robust effect on tumor growth was confirmed in vivo in a large series of cell line-derived xenograft and patient-derived xenograft mouse models. Mechanistic studies, including single-cell RNA sequencing of multicellular patient HCC tumorspheres, suggested that CLDN1 regulates tumor stemness, metabolism, oncogenic signaling and perturbs the tumor immune microenvironment (Fig.3). The results provide the rationale for targeting CLDN1 in HCC and pave the way for the clinical development of CLDN1-specific mAbs for the treatment of advanced HCC.
References
[1]Huang J, Liu D, Wang Y, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734-745. (IF=31.793)
[2]Yu X, Orr CM, Chan HTC, et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature. 2023;614(7948):539-547. (IF=69.504)
[3]Roehlen N, Muller M, Nehme Z, et al. Treatment of HCC with claudin-1-specific antibodies suppresses carcinogenic signaling and reprograms the tumor microenvironment. J Hepatol. 2023;78(2):343-355. (IF=30.083)
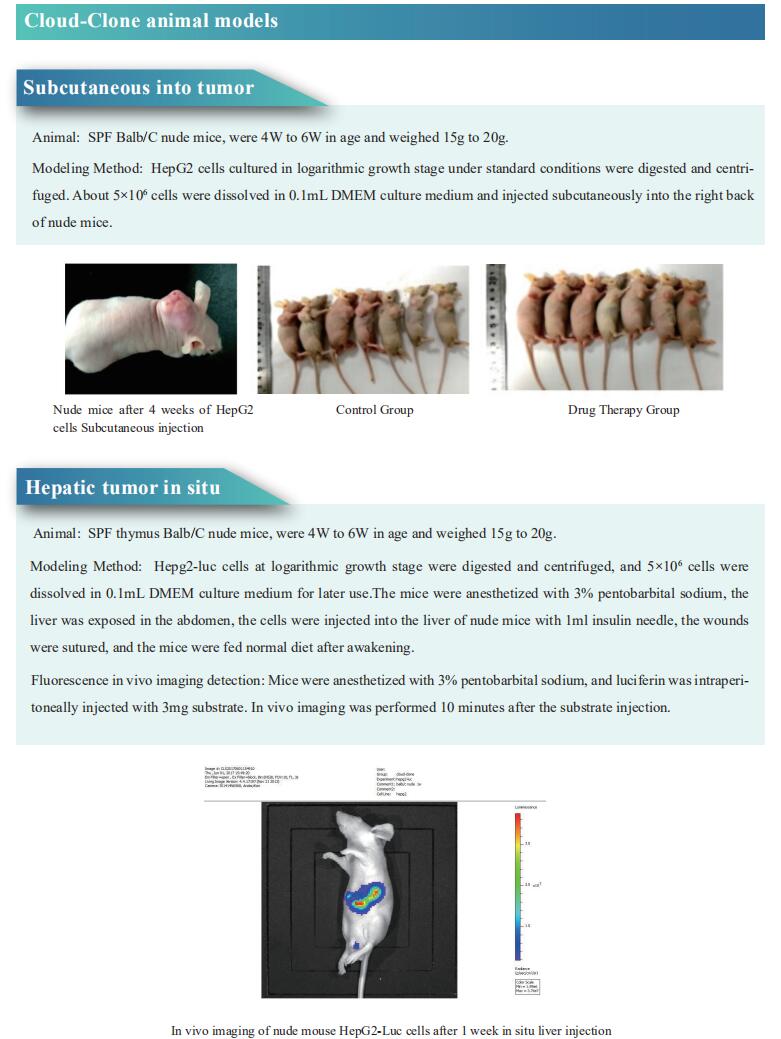
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators and the above-mentioned PD-1, PD-L1, CD40, 4-1BB, CLDN1 and other related products, which can help scientific researchers to conduct cancer-related research.