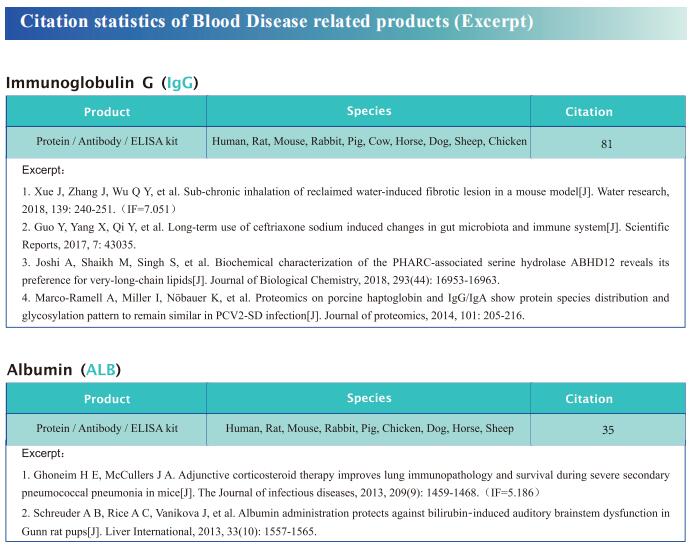
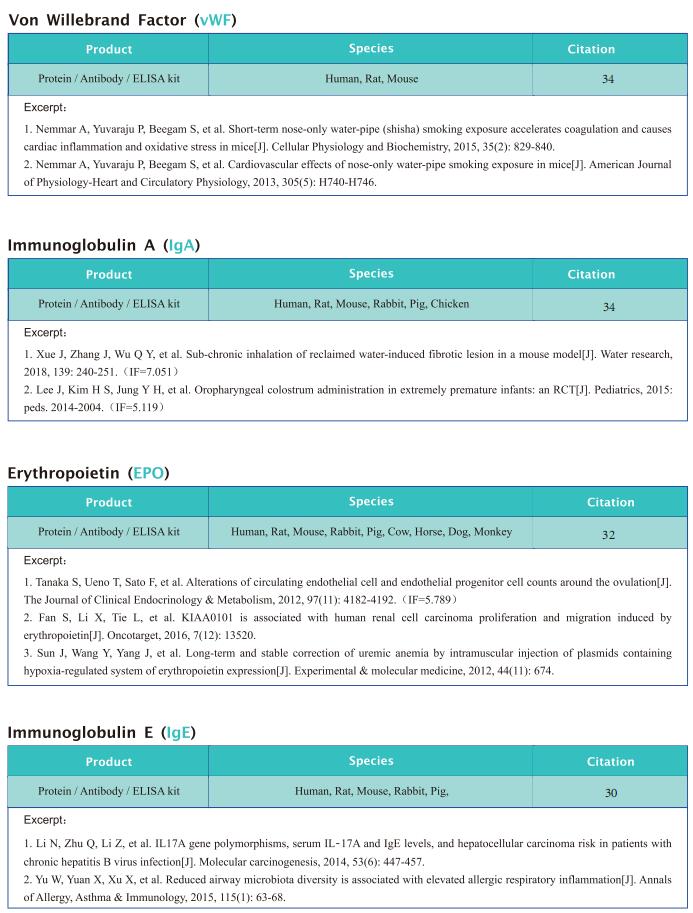
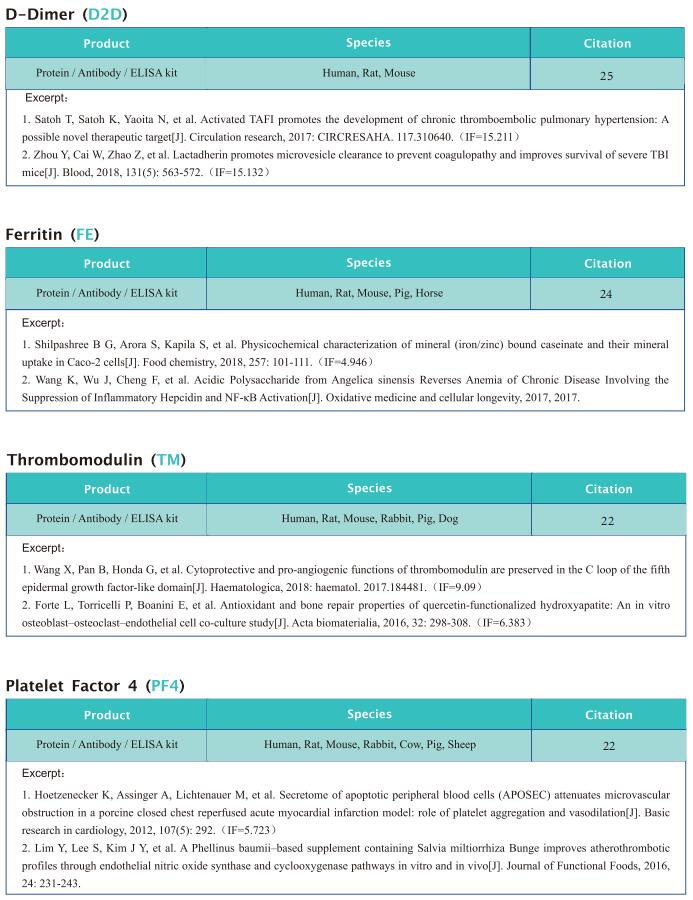
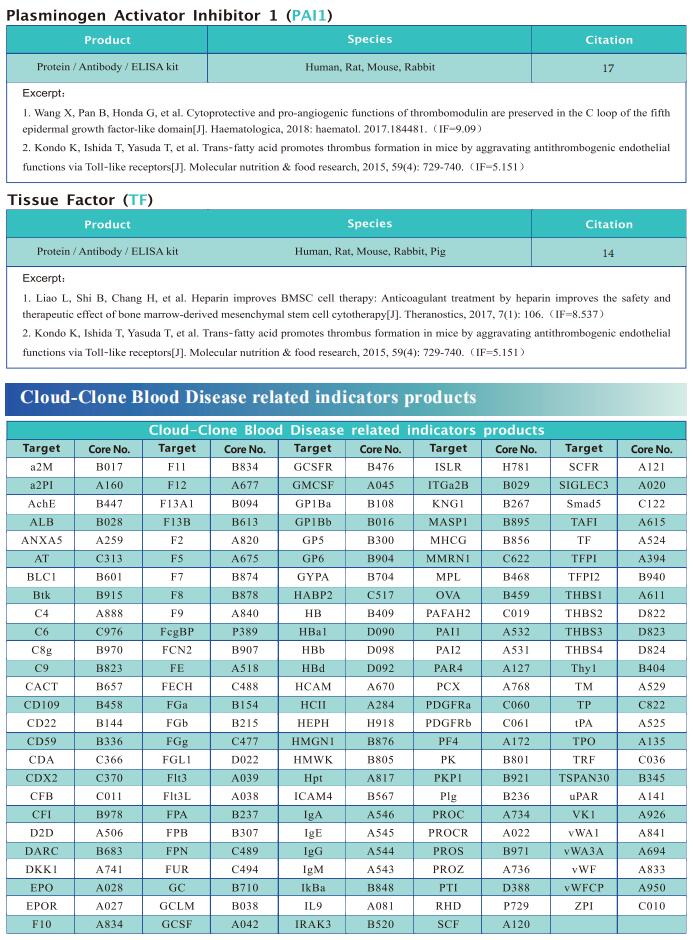
New progress in research on anemia related diseases
Anemia, a disease characterized by decreased numbers of circulating red blood cells and/or hemoglobin, is a major public health problem worldwide, with a prevalence greater than 30%. Recently, this disease has become more prominent due to an aging population and a higher incidence of chronic diseases and cancers. In humans, anemia can be caused by different pathophysiologic processes; for example, primary anemia is caused by hematopoietic system abnormalities, while secondary anemia is induced by chronic diseases, kidney diseases, and cancers, among others. As the most common hematological manifestation, anemia can affect recovery from the disease itself. Therefore, systematic and in-depth research on the pathological mechanism of anemia may contribute to the treatment of related diseases to improve the prognosis of patients.
1. Activation of γ-globin expression by hypoxia-inducible factor 1α
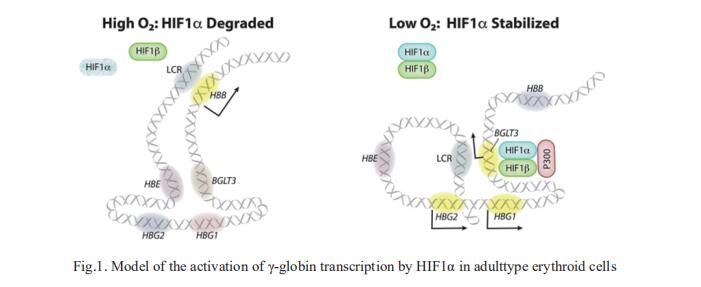
Around birth, globin expression in human red blood cells (RBCs) shifts from γ-globin to β-globin, resulting in fetal hemoglobin (HbF, α2γ2) being gradually replaced by adult hemoglobin (HbA, α2β2). This process has motivated innovative approaches to treating sickle cell disease (SCD) and β-thalassemia by increasing HbF in postnatal RBCs. Mitchell J. Weiss, Department of Hematology, St Jude Children’s Research Hospital, Memphis, TN, USA, and his team provided therapeutically relevant insights into globin gene switching obtained through a CRISPR/Cas9 screen for ubiquitin-proteasome components that regulate HbF expression[1]. In RBC precursors, depletion of the von Hippel-Lindau (VHL) E3 ubiquitin ligase stabilized its ubiquitination target, hypoxia-inducible factor 1-alpha (HIF1α), to induce γ-globin gene transcription. Mechanistically, HIF1α-HIF1β heterodimers bound cognate DNA elements in BGLT3 (Fig.1), a long noncoding RNA gene located 2.7 kb downstream of the tandem γ-globin genes HBG1 and HBG2. This was followed by recruitment of transcriptional activators, chromatin opening, and increased long-range interactions between the γ-globin genes and their upstream enhancer. Similar induction of HbF occurred with hypoxia or with inhibition of prolyl hydroxylase domain enzymes that target HIF1α for ubiquitination by the VHL E3 ubiquitin ligase. These findings link globin gene regulation with canonical hypoxia adaptation, provide a mechanism for HbF induction during stress erythropoiesis, and suggest a novel therapeutic approach for β-hemoglobinopathies.
2. Carbon Dots as a Potential Therapeutic Agent for the Treatment of Cancer-Related Anemia
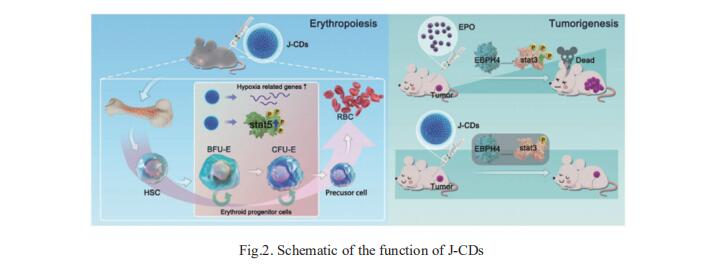
Due to the adverse effects of erythropoietin (EPO) on cancer patient survival, it is necessary to develop new agents that can be used to efficiently manage and treat cancer-related anemia. Shijie Zhang, School of Life Sciences, Zhengzhou University, China, and his team designed, synthesized, and characterized a novel distinctive carbon dots, J-CDs, derived from jujube[2]. Based on the obtained results, this material comprises sp2 and sp3 carbon atoms, as well as oxygen/nitrogen-based groups, and it specifically promotes the proliferation of erythroid cells by stimulating the self-renewal of erythroid progenitor cells in vitro and in vivo. Moreover, J-CDs have no discernible effects on tumor proliferation and metastasis, unlike EPO. Transcriptome profiling suggests that J-CDs upregulate the molecules involved in hypoxia response, and they also significantly increase the phosphorylation levels of STAT5 (Fig.2), the major transducer of signals for erythroid progenitor cell proliferation. Overall, this study demonstrates that J-CDs effectively promote erythrocyte production without affecting tumor proliferation and metastasis; thus, they may be promising agents for the treatment of cancer-related anemia.
3. Single-cell analysis highlights a population of Th17-polarized CD4+ naïve T cells showing IL6/JAK3/STAT3 activation in pediatric severe aplastic anemia
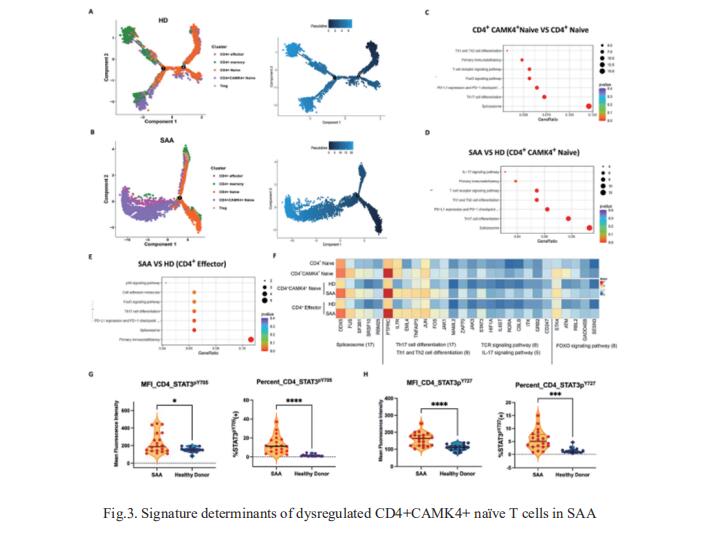
Acquired aplastic anemia (AA) is recognized as an immune-mediated disorder resulting from active destruction of hematopoietic cells in bone marrow (BM) by effector T lymphocytes. To uncover dysfunctional mechanisms acting within the heterogeneous marrow-infiltrating immune environment and examine their pathogenic interplay with the hematopoietic stem/progenitor pool, Yingchi Zhang, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China, and his team exploited single-cell mass cytometry for BM mononuclear cells of severe AA (SAA) patients pre- and post-immunosuppressive therapy, in contrast to those of healthy donors[3]. Alignment of BM cellular composition with hematopoietic developmental trajectories revealed potential functional roles for non-canonically activated CD4+ naïve T cells in newly-diagnosed pediatric cases of SAA (Fig.3). Furthermore, single-cell transcriptomic profiling highlighted a population of Th17-polarized CD4+CAMK4+ naïve T cells showing activation of the IL-6/JAK3/STAT3 pathway, while gene signature dissection indicated a predisposition to proinflammatory pathogenesis. Retrospective validation from their SAA cohort of 231 patients revealed high plasma levels of IL-6 as an independent risk factor of delayed hematopoietic response to antithymocyte globulin-based immunosuppressive therapy. Thus, IL-6 warrants further investigation as a putative therapeutic target in SAA.
References
[1]Feng R, Mayuranathan T, Huang P, et al. Activation of γ-globin expression by hypoxia-inducible factor 1α [J]. Nature. 2022,610(7933):783-790. (IF=69.504)
[2]Xu Y, Wang B, Zhang M, et al. Carbon Dots as a Potential Therapeutic Agent for the Treatment of Cancer-Related Anemia [J]. Adv Mater. 2022,34(19):e2200905. (IF=32.086)
[3]Zhang J, Liu T, Duan Y, et al. Single-cell analysis highlights a population of Th17-polarized CD4+ naïve T cells showing IL6/JAK3/STAT3 activation in pediatric severe aplastic anemia [J]. J Autoimmun. 2023,136:103026. (IF=14.511)
Cloud-Clone not only provides animal models of anemia related diseases such as iron deficiency anemia, acute hemorrhagic anemia, aplastic anemia, hemolytic anemia, etc., but also has the aforementioned hemoglobin, HIF1 α, STAT5 and IL-6/JAK3/STAT3 pathway related indicator detection products, which can help researchers to carry out anemia related research.