New progress in research on esophageal cancer
Esophageal cancer (EC) is the eighth most common cancer globally and has the sixth worst prognosis because of its aggressiveness and poor survival. The worldwide incidence of EC has been increasing over the past 3 decades, with some variation in the two main histological subtypes. Squamous cell carcinoma (SCC) is still the more common type of EC globally, with 79% of all SCC cases occurring in Asia . However, Western industrialized nations have experienced a rapid increase of esophageal adenocarcinoma (AC). EAC typically has a better overall median survival than ESCC, particularly in early stage disease. Despite countless improvements in diagnostic and treatment techniques over the past three decades, the prognosis for EC remains poor. Recently, multiple papers have reported on EC related studies, which may provide assistance for the early diagnosis and treatment of EC.
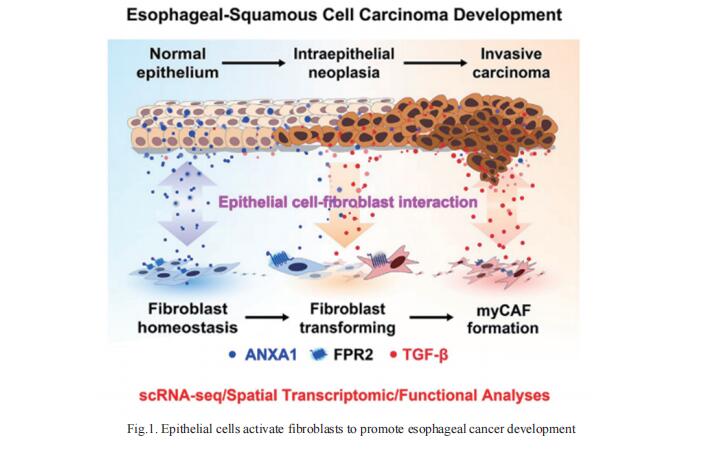
1. Epithelial cells activate fibroblasts to promote esophageal cancer development
ESCC develops through multistage epithelial cancer formation, i.e., from normal epithelium, low- and high-grade intraepithelial neoplasia to invasive carcinoma. However, how the precancerous lesions progress to carcinoma remains elusive. Chen Wu, Department of Etiology and Carcinogenesis, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China, and his team reported a comprehensive singlecell RNA sequencing and spatial transcriptomic study of 79 multistage esophageal lesions from 29 patients with ESCC[1]. They revealed a gradual and significant loss of ANXA1 expression in epithelial cells due to its transcription factor KLF4 suppression along the lesion progression. They demonstrated that ANXA1 is a ligand to formyl peptide receptor type 2 (FPR2) on fibroblasts that maintain fibroblast homeostasis (Fig.1). Loss of ANXA1 leads to uncontrolled transformation of normal fibroblasts into cancer-associated fibroblasts (CAFs), which can be enhanced by secreted TGF-b from malignant epithelial cells. Given the role of CAFs in cancer, the study underscores ANXA1/FPR2 signaling as an important crosstalk mechanism between epithelial cells and fibroblasts in promoting ESCC.
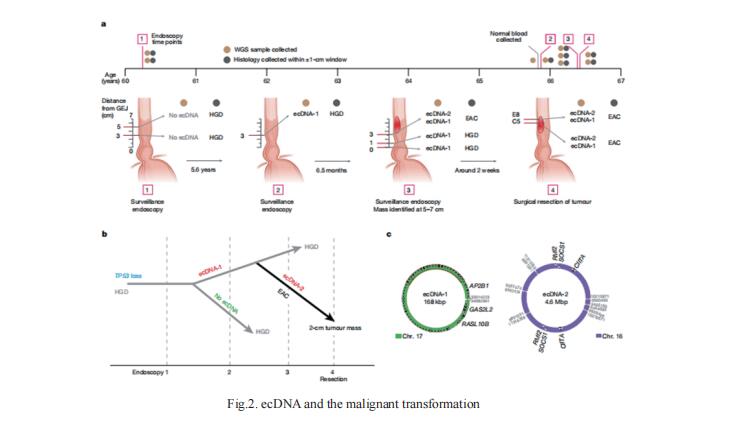
2. Extrachromosomal DNA in the cancerous transformation of Barrett’s oesophagus
Oncogene amplifcation on extrachromosomal DNA (ecDNA) drives the evolution of tumours and their resistance to treatment, and is associated with poor outcomes for patients with cancer. Barrett’s esophagus, a metaplastic transformation from the normal squamous mucosa of the esophagus to a columnar lining, is the only known precursor for esophageal adenocarcinoma. To better understand the development of ecDNA, Paul S. Mischel, Department of Pathology, Stanford University School of Medicine, USA, and his team analysed whole-genome sequencing (WGS) data from patients with oesophageal adenocarcinoma (EAC) or Barrett’s oesophagus[2]. The frequency of ecDNA increased between Barrett’s-oesophagus-associated early-stage (24%) and late-stage (43%) EAC, suggesting that ecDNA is formed during cancer progression. In biopsies that were collected before cancer diagnosis, higher levels of ecDNA were present in samples from patients who later developed EAC than in samples from those who did not. They found that ecDNAs contained diverse collections of oncogenes and immunomodulatory genes (Fig.2). Furthermore, ecDNAs showed increases in copy number and structural complexity at more advanced stages of disease. These fndings show that ecDNA can develop early in the transition from high-grade dysplasia to cancer, and that ecDNAs progressively form and evolve under positive selection.
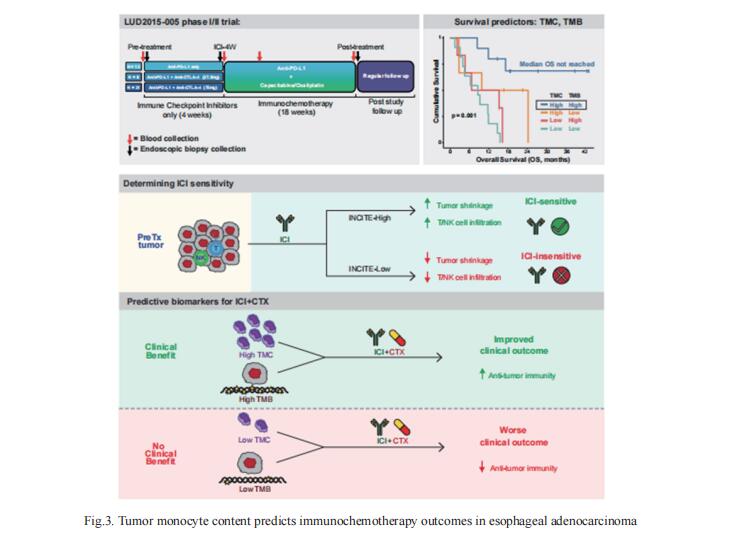
3. Tumor monocyte content predicts immunochemotherapy outcomes in esophageal adenocarcinoma
For inoperable EAC, identifying patients likely to benefit from recently approved immunochemotherapy (ICI+CTX) treatments remains a key challenge. Xin Lu, Ludwig Institute for Cancer Research, University of Oxford, UK, and his team addressed this using a uniquely designed window-of-opportunity trial (LUD2015-005), in which 35 inoperable EAC patients received first-line immune checkpoint inhibitors for four weeks (ICI-4W), followed by ICI+CTX[3]. Comprehensive biomarker profiling, including generation of a 65,000-cell single-cell RNA-sequencing atlas of esophageal cancer, as well as multi-timepoint transcriptomic profiling of EAC during ICI-4W, reveals a novel T cell inflammation signature (INCITE) whose upregulation correlates with ICI-induced tumor shrinkage. Deconvolution of pre-treatment gastro-esophageal cancer transcriptomes using the single-cell atlas identifies high tumor monocyte content (TMC) as an unexpected ICI+CTX-specific predictor of greater overall survival (OS) in LUD2015-005 patients and of ICI response in prevalent gastric cancer subtypes from independent cohorts (Fig.3). Tumor mutational burden is an additional independent and additive predictor of LUD2015-005 OS. TMC can improve patient selection for emerging ICI+CTX therapies in gastro-esophageal cancer.
References
[1]Chen Y, Zhu S, Liu T, et al. Epithelial cells activate fibroblasts to promote esophageal cancer development [J]. Cancer Cell. 2023;41(5):903-918.e8. (IF=50.3)
[2]Luebeck J, Ng AWT, Galipeau PC, et al. Extrachromosomal DNA in the cancerous transformation of Barrett's oesophagus [J]. Nature. 2023;616(7958):798-805. (IF=64.8)
[3]Carroll TM, Chadwick JA, Owen RP, et al. Tumor monocyte content predicts immunochemotherapy outcomes in esophageal adenocarcinoma [J]. Cancer Cell. 2023;41(7):1222-1241.e7. (IF=50.3)
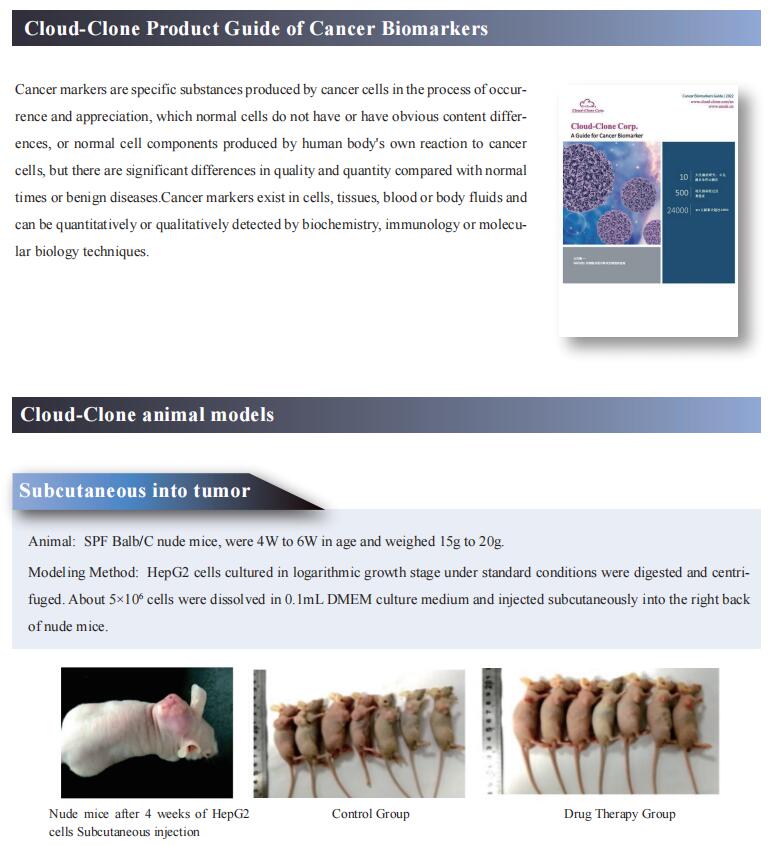
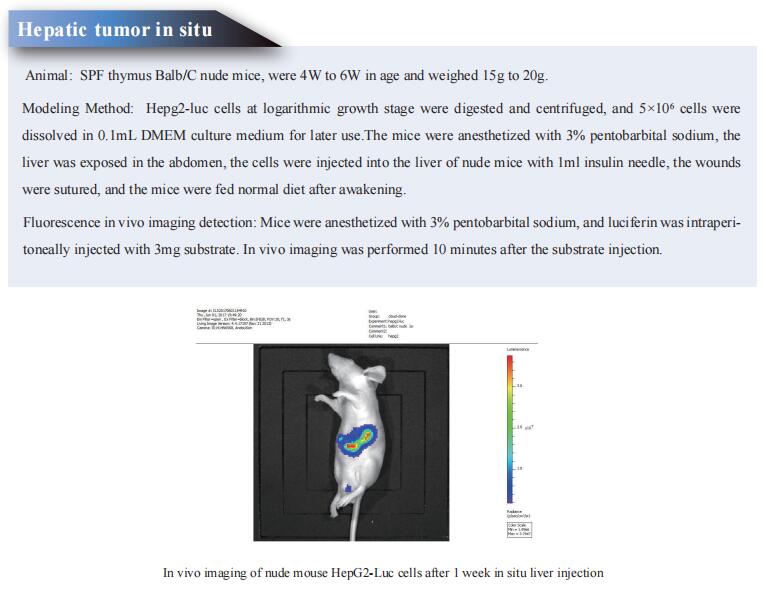
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators, the above-mentioned KLF4, ANXA1, FPR2, TGF-b and other related products, which can help scientific researchers to conduct cancer-related research.