New progress in research on myocardial infarction
Myocardial infarction (MI) is a term for an event of heart attack. MI occurs when blood stops flowing properly to a part of the heart, and the heart muscle is injured because of lack of oxygen supply. And one of the coronary arteries which supplies blood to the heart develops a blockage due to an unstable buildup of plaques, white blood cells, cholesterol and fat. If the event becomes serious then it is called as acute myocardial infarction (AMI). Because adult cardiomyocytes have little regenerative capacity, myocardial tissue lost in MI is replaced with fibrotic tissue, leading to cardiac dysfunction and Heart failure (HF). Recently, a number of papers have reported the research on cardiomyocytes repair after MI, which may provide help for the prevention and treatment of heart disease.
1. Direct Reprogramming Improves Cardiac Function and Reverses Fibrosis in Chronic Myocardial Infarction
Because adult cardiomyocytes have little regenerative capacity, resident cardiac fibroblasts (CFs) synthesize extracellular matrix after MI to form fibrosis, leading to cardiac dysfunction and HF. Masaki Ieda, Department of Cardiology, Faculty of Medicine, University of Tsukuba, Japan, and his team used mice overexpressing cardiac transcription factor [Mef2c/Gata4/Tbx5/Hand2 (MGTH)] to study the effect of cardiac reprogramming events on chronic MI[1]. The overexpression of MGTH, can directly reprogram CFs into induced cardiomyocytes (iCMs), significantly improved myocardial contraction and reduced fibrosis in chronic MI. Singlecell RNA sequencing demonstrated that resident CFs consisted of 7 subclusters, in which the profibrotic CF population increased under chronic MI. Cardiac reprogramming suppressed fibroblastic gene expression in chronic MI by means of conversion of profibrotic CFs to a quiescent antifibrotic state (Fig.1). MGTH overexpression induced antifibrotic effects partly by suppression of Meox1, a central regulator of fibroblast activation. These results demonstrate that cardiac reprogramming could repair chronic MI by means of myocardial regeneration and reduction of fibrosis. These findings present opportunities for the development of new therapies for chronic MI and HF.
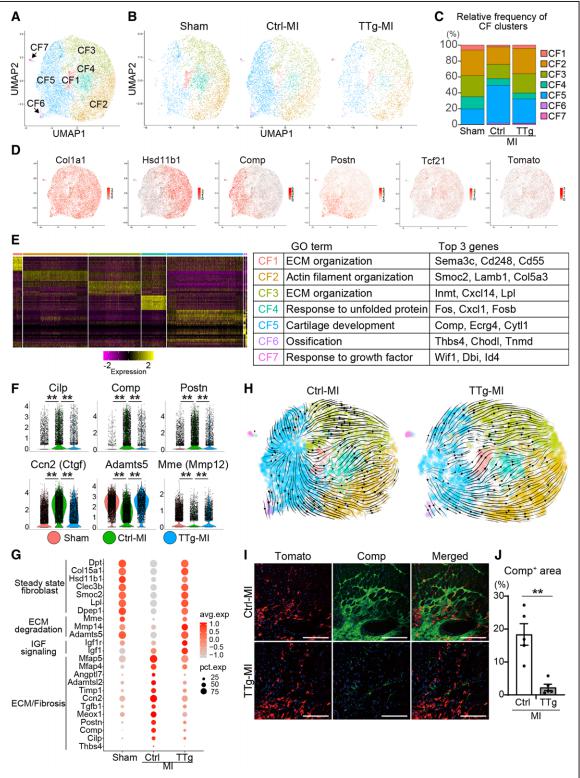
Fig.1 Cardiac reprogramming converted profibrotic CFs to a quiescent state in chronic myocardial infarction
2. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in MI
Mesenchymal stem cells (MSCs) delivered into the post-ischemic heart milieu have a low survival and retention rate, thus restricting the cardioreparative efficacy of MSC-based therapy. Ling Tao, Department of Cardiology, Xijing Hospital, the Fourth Military Medical University, China, and his team found that excessive branched-chain amino acid (BCAA) accumulation was disadvantageous to the retention and cardioprotection of intramyocardially injected MSCs[2]. Discovery-driven experiments revealed that BCAA at pathological levels sensitized MSCs to stress-induced cell death and premature senescence via accelerating the loss of histone 3 lysine 9 trimethylation (H3K9me3). A novel mTORC1/DUX4/KDM4E axis was identified as the cause of BCAA-induced H3K9me3 loss and adverse phenotype acquisition (Fig.2). Enhancing BCAA catabolic capability in MSCs via genetic/pharmacological approaches greatly improved their adaptation to the high BCAA milieu and strengthened their cardioprotective efficacy. The present study emphasizes that enhancing BCAA catabolism in MSCs via the appropriate approaches might be a feasible strategy to improve the adaptation of MSCs to the ischemic myocardial milieu and may thus optimize the cardioreparative efficacy of MSC-based therapy.
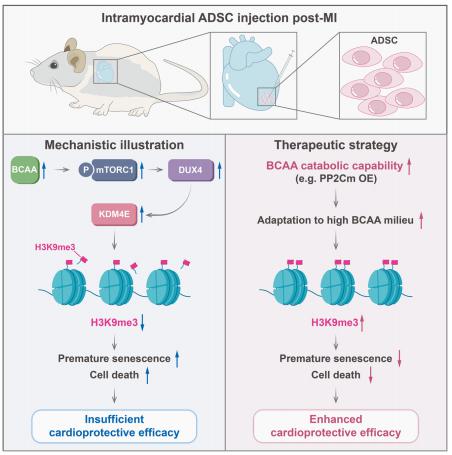
Fig.2 Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in MI
3. Novel Cardiokine GDF3 Predicts Adverse Fibrotic Remodeling After MI
Jean-Sébastien Hulot, Paris Cardiovascular Research Center, INSERM, Université de Paris, France, and his team explored whether the expansion of CFs after MI can be regulated through a paracrine action of cardiac stromal cells[3]. Cardiac stromal PW1+ cells undergo a change in paracrine behavior after MI, and the conditioned media from these cells induced a significant increase in the proliferation of fibroblasts. They identified a total of 12 candidates as secreted proteins overexpressed by cardiac PW1+ cells after MI. Among these factors, GDF3, a member of the TGF-β family, was markedly upregulated in the ischemic hearts. Conditioned media specifically enriched with GDF3 induced fibroblast proliferation at a high level by stimulation of activin-receptor–like kinases. In line with the secretory nature of this protein, they next found that GDF3 can be detected in mice and human plasma samples, with a significant increase in the days after MI (Fig.3). In humans, higher GDF3 circulating levels were significantly associated with an increased risk of adverse remodeling 6 months after MI, including lower left ventricular ejection fraction and a higher proportion of akinetic segments. Their findings define a mechanism for the profibrotic action of cardiac stromal cells through secreted cardiokines, such as GDF3, a candidate marker of adverse fibrotic remodeling after MI.
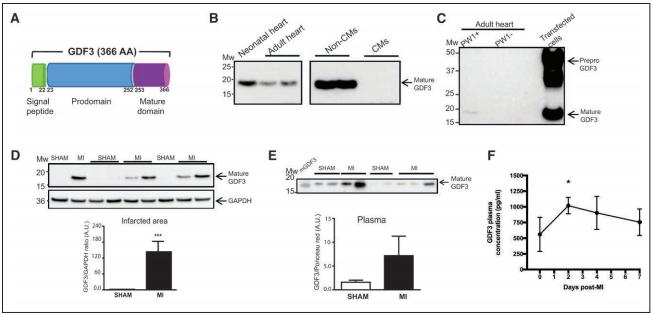
Fig.3 GDF3 expression is upregulated in the plasma and infarcted area of mouse heart after MI
4. RNA-binding Protein LIN28a Regulates New Myocyte Formation in the Heart via lncRNA-H19
Developmental cardiac tissue holds remarkable capacity to regenerate after injury and consists of regenerative mononuclear and diploid cardiomyocytes (MNDCMs). Upon maturation, MNDCMs become binucleated or polyploid and exit the cell cycle. However, whether reprogramming metabolism promotes persistence of regenerative MNDCMs enhancing cardiac function and repair after injury is unknown. Mohsin Khan, Department of Cardiovascular Sciences, Lewis Katz School of Medicine, Temple University, USA, and his team identified a novel role for RNA-binding protein LIN28a in cardiac repair following injury[4]. LIN28a was found as primarily active during cardiac development and rapidly decreases after birth. In the adult heart, LIN28a overexpression attenuated CM apoptosis, enhanced cell cycle activity, cardiac function, and survival in mice 12 weeks after myocardial infarction compared to WT littermate controls. Alternatively, LIN28a small molecule inhibitor attenuated pro-reparative effects of LIN28a on the heart. Mechanistically, Neonatal rat ventricular myocytes overexpressing LIN28a showed increased glycolysis, ATP production and levels of metabolic enzymes compared to control. LIN28a immunoprecipitation followed by RNA sequencing (RIPseq) in CMs isolated from LIN28aoverexpressing hearts after injury identified lncRNA-H19 as its most significantly alteredtarget (Fig.4). Ablation of lncRNA-H19 blunted LIN28a-induced enhancement on CM metabolismand cell cycle activity. LIN28a promotes cell cycle activity and augments cardiac structure and function by reprogramming CM metabolism towards glycolysis dependent upon RNA-binding to lncRNA-H19 after myocardial injury.
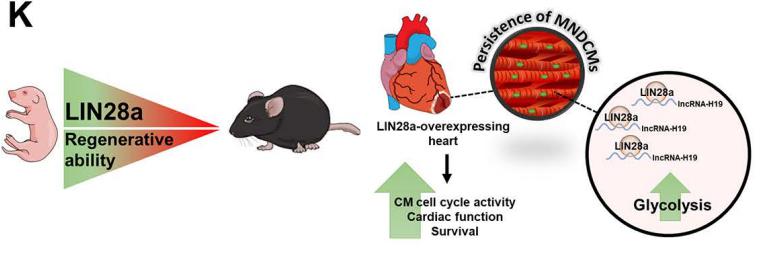
Fig.4 RNA-binding Protein LIN28a Regulates New Myocyte Formation in the Heart via lncRNA-H19
Cloud-Clone can not only provide a variety of cardiovascular system disease models, including myocardial infarction, arrhythmia, myocardial hypertrophy, myocarditis, heart failure, covering common cardiovascular system diseases. We also have various commonly used indicators of cardiovascular system signaling pathways and the above mTORC1, GDF3, LIN28a and other related products, which can help the majority of scientific researchers to carry out research on cardiovascular system diseases.
References
[1]Tani H, Sadahiro T, Yamada Y, et al. Direct Reprogramming Improves Cardiac Function and Reverses Fibrosis in Chronic Myocardial Infarction [J]. Circulation. 2022;10.1161/CIRCULATIONAHA.121.058655. (IF=39.918)
[2]Zhang F, Hu G, Chen X, et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction [J]. Signal Transduct Target Ther. 2022;7(1):171. (IF=38.104)
[3]Masurkar N, Bouvet M, Logeart D, et al. Novel Cardiokine GDF3 Predicts Adverse Fibrotic Remodeling After Myocardial Infarction [J]. Circulation. 2022;10.1161/CIRCULATIONAHA.121.056272. (IF=39.918)
[4]Rigaud VOC, Hoy R, Kurian J, et al. RNA-binding Protein LIN28a Regulates New Myocyte Formation in the Heart via lncRNA-H19 [J]. Circulation. 2022;10.1161/CIRCULATIONAHA.122.059346. (IF=39.918)


