New progress in research on retinal diseases
The retina is a visual nerve endings extending outward from the brain. Its function is to receive external light stimuli, convert them into physiological stimuli through photochemical reactions, and transmit them to the central nervous system to generate vision. Any retinal disease can cause visual impairment, and the degree of damage varies depending on the severity and location of the lesion. Common retinal diseases include diabetic retinopathy (DR), retinal vascular occlusion, retinitis pigmentosa, age-related macular degeneration (AMD), glaucoma and retinopathy of prematurity. Recently, multiple studies have reported on retinal diseases, which may provide assistance for the prevention and treatment of related diseases.
1. Bioprinted 3D Outer Retina Barrier Uncovers RPEdependent Choroidal Phenotype in Advanced Macular Degeneration
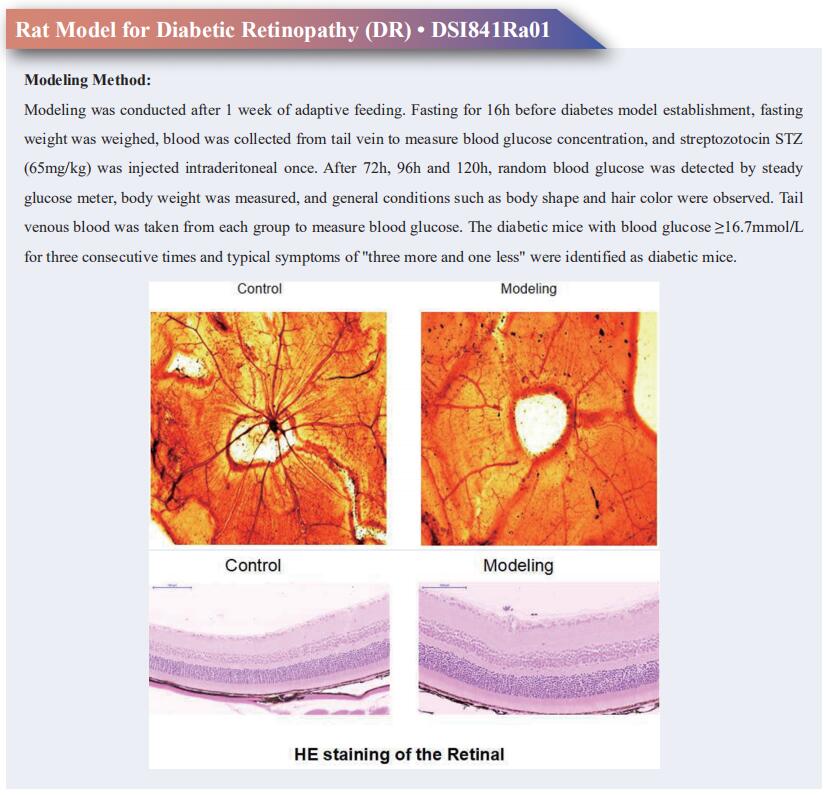
Age-related macular degeneration (AMD), a leading cause of blindness, initiates in the outer-blood-retina-barrier (oBRB) formed by Retinal pigment epithelium (RPE), Bruch’s membrane, and choriocapillaris. The mechanism of AMD initiation and progression remain poorly understood due to the lack of physiologically relevant oBRB models. Kapil Bharti, National Eye Institute, National Institutes of Health, USA, and his team engineered a native-like 3D-oBRB tissue by bioprinting endothelial cells, pericytes, and fibroblasts on the basal side of a biodegradable scaffold and establishing an RPE monolayer on top[1]. In this 3D-oBRB, a fully-polarized RPE monolayer with apical processes and basal infoldings provides barrier resistance, induces fenestration and choroid-specific gene expression in the choriocapillaris, and supports the formation of a Bruch’s-like membrane that allows tissue integration in rat eyes. Complement activation in the 3D-oBRB triggers dry-AMD phenotypes (including subRPE drusen and choriocapillaris degeneration), and hypoxia activated HIF-α induces wet-AMD phenotypes (choriocapillaris neovascularization) (Fig.1). Anti-VEGF drug treatment suppresses neovascularization -validating this model for clinical translation and drug discovery.
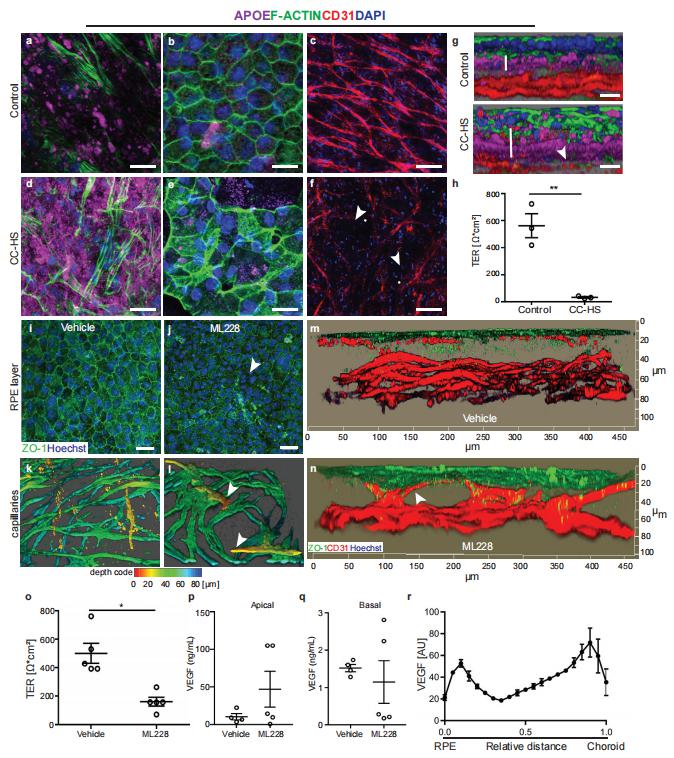
Fig.1 RPE dependent choroid degeneration in dry and wet AMD models of 3D-oBRB
2. CEP162 deficiency causes human retinal degeneration
Defects in primary or motile cilia result in a variety of human pathologies, and retinal degeneration is frequently associated with these so-called ciliopathies. Hanno J. Bolz, Senckenberg Centre for Human Genetics, Germany, and his team found that homozygosity for a truncating variant in CEP162, a centrosome and microtubule-associated protein required for transition zone assembly during ciliogenesis and neuronal differentiation in the retina, caused late-onset retinitis pigmentosa in 2 unrelated families[2]. The mutant CEP162-E646R*5 protein was expressed and properly localized to the mitotic spindle, but it was missing from the basal body in primary and photoreceptor cilia. This impaired recruitment of transition zone components to the basal body and corresponded to complete loss of CEP162 function at the ciliary compartment, reflected by delayed formation of dysmorphic cilia. In contrast, shRNA knockdown of Cep162 in the developing mouse retina increased cell death, which was rescued by expression of CEP162-E646R*5, indicating that the mutant retains its role for retinal neurogenesis (Fig.2). Human retinal degeneration thus resulted from specific loss of the ciliary function of CEP162.
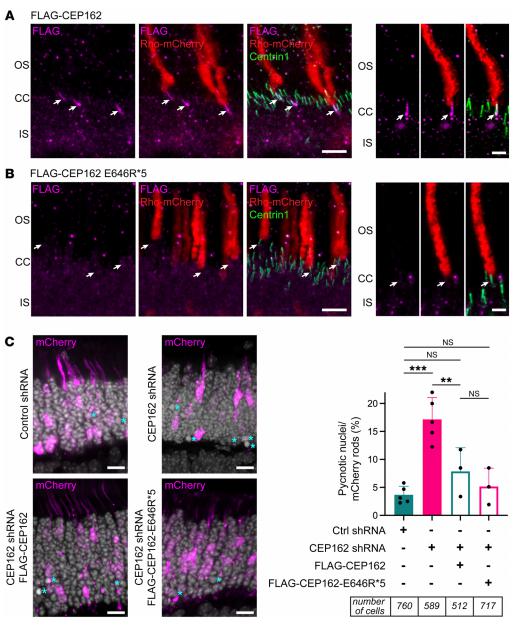
Fig.2 CEP162-E646R*5 mutant protein does not localize to centrioles in adult mouse rod photoreceptors but does participate in retinal neurogenesis
3. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration
The development of therapeutics for effective treatments of retinal diseases is significantly constrained by various biological barriers. Jui-Yang Lai, Department of Biomedical Engineering, Chang Gung University, Taiwan, and his team reported a nanomedicine strategy to develop nanotherapeutics featured with not only high retinal permeability but also sustained bioactive delivery[3]. Specifically, the nanotherapeutics are rationally designed via aminolysis of resveratrol-encapsulated polycaprolactone nanoparticles (R@PCL NPs), followed by the formation of amide linkages with carboxyl-terminated transacting activator of transcription cell penetrating peptide (T) and metformin (M). The R@PCL-T/M NP nanotherapeutics are demonstrated in vitro to possess persistent drug release profiles, good ocular biocompatibility, and potent bioactive activities for targeting prevailing risk factors associated with retinal diseases. In vivo studies indicate that single-dose intravitreal administration of the R@PCL-T/M NPs can effectively improve retinal permeability (~15-fold increase), prevent loss of endogenous antioxidants, and suppress the growth of abnormal vessels in the retina with macular degeneration for 56 days (Fig.3). This high treatment efficacy can be ascribed to the enhanced retinal permeability of the nanotherapeutics in conjunction with the sustained pharmacological activity of the dual drugs (R and M) in the retinal pigment epithelial region. These findings show a great promise for the development of pharmacological nanoformulations capable of targeting the retina and thereby treating complex posterior segment diseases with improved efficacies.
Fig.3 Effect of R@PCL-T/M NPs on the inhibition of VEGF-A165-induced angiogenesis and vascular development in chick chorioallantoic membrane
4. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates
Lipid nanoparticle (LNP)–based mRNA delivery holds promise for the treatment of inherited retinal degenerations. Currently, LNP-mediated mRNA delivery is restricted to the retinal pigment epithelium (RPE) and Müller glia. LNPs must overcome ocular barriers to transfect neuronal cells critical for visual phototransduction, the photoreceptors (PRs). Gaurav Sahay, Department of Pharmaceutical Sciences, College of Pharmacy, Robertson Life Sciences Building, Oregon State University, USA, and his team used a combinatorial M13 bacteriophage-based heptameric peptide phage display library for the mining of peptide ligands that target PRs[4]. They identified the most promising peptide candidates resulting from in vivo biopanning. Dye-conjugated peptides showed rapid localization to the PRs. LNPs decorated with the top-performing peptide ligands delivered mRNA to the PRs, RPE, and Müller glia in mice. This distribution translated to the nonhuman primate eye, wherein robust protein expression was observed in the PRs, Müller glia, and RPE (Fig.4). Overall, they have developed peptide-conjugated LNPs that can enable mRNA delivery to the neural retina, expanding the utility of LNP-mRNA therapies for inherited blindness.
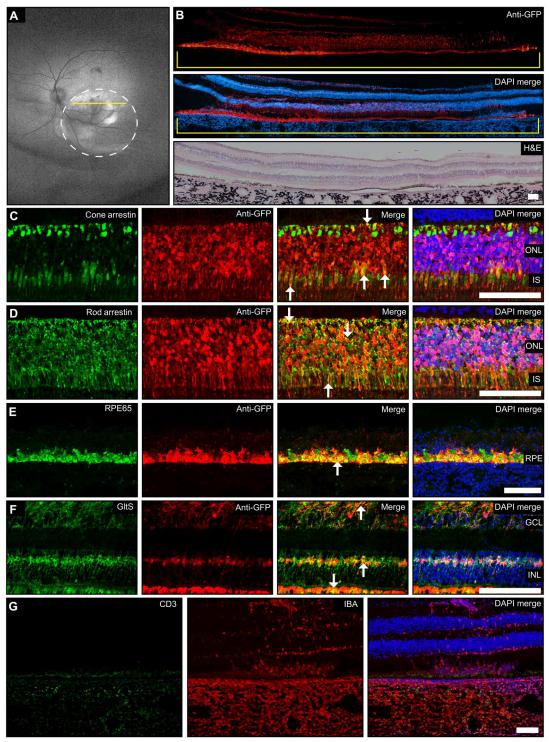
Fig.4 MH42-conjugated LNPs mediate expression in the neuralretina after subretinal administration in the NHP
References
[1]Song MJ, Quinn R, Nguyen E, et al. Bioprinted 3D outer retina barrier uncovers RPE-dependent choroidal phenotype in advanced macular degeneration [J]. Nat Methods. 2023,20(1):149-161. (IF=47.990)
[2]Nuzhat N, Van Schil K, Liakopoulos S, et al. CEP162 deficiency causes human retinal degeneration and reveals a dual role in ciliogenesis and neurogenesis [J]. J Clin Invest. 2023,133(8):e161156. (IF=19.456)
[3]Nguyen DD, Luo LJ, Yang CJ, Lai JY. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration [J]. ACS Nano. 2023,17(1):168-183. (IF=18.027)
[4]Herrera-Barrera M, Ryals RC, Gautam M, et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates [J]. Sci Adv. 2023,9(2):eadd4623. (IF=14.957)
Cloud-Clone can provide animal models of various intraocular diseases, including diabetic retinopathy, retinal ischemia reperfusion injury, retinal artery/vein occlusion, retinopathy of prematurity, retinal detachment and other common intraocular diseases. We also have various detection indicators of ocular diseases and primary cell products such as retinal astrocytes, retinal pigment epithelial cells, retinal ganglion cells, ocular choroid fibroblasts, etc., which can help the majority of scientific researchers to carry out research on the treatment of ocular diseases.