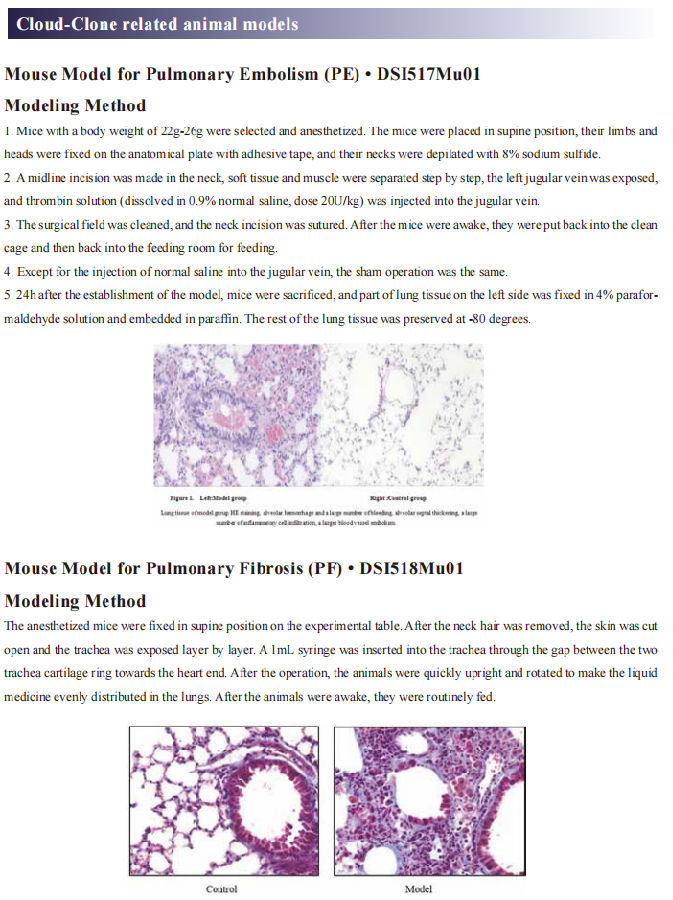
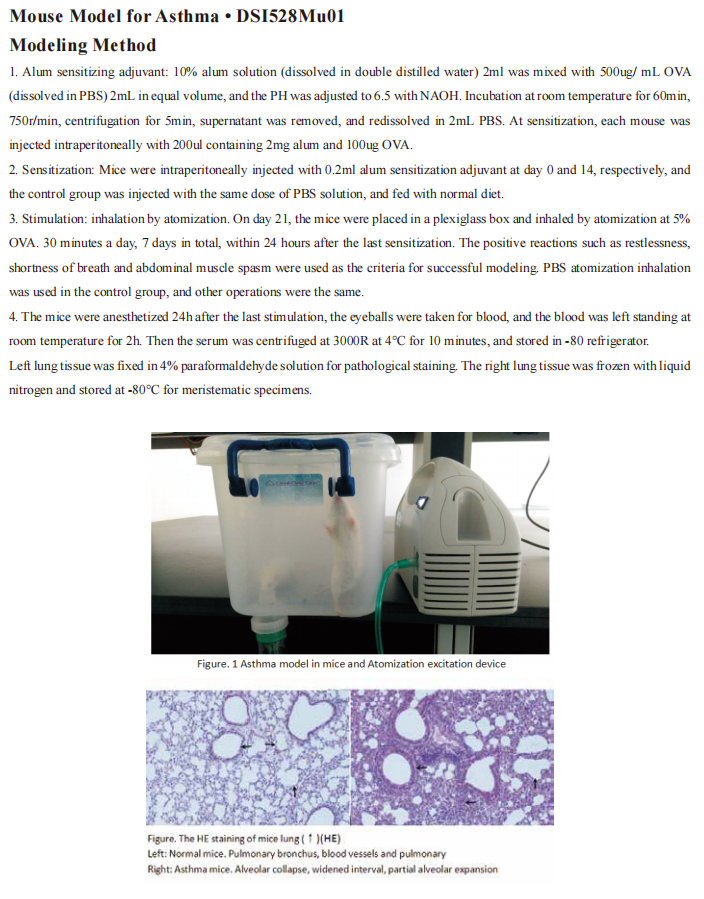
New progress in the pathogenesis of pulmonary fibrosis
Pulmonary fibrosis (PF) is a major category of terminal changes of lung diseases characterized by proliferation of fibroblasts, accumulation of a large amount of extracellular matrix, accompanied by inflammatory damage and destruction of tissue structure, namely, structural abnormalities caused by abnormal repair of normal alveolar tissue after damage. The cause of most PF is unknown and is called idiopathic interstitial pneumonia. In idiopathic interstitial pneumonia, the most common disease type with PF lesions as the main manifestation is idiopathic PF, which is an interstitial lung disease that can lead to progressive loss of lung function.
New progress in the research on the pathogenesis of PF
Most PF patients have a poor prognosis. Although recently available anti-fibrotic treatment modalities, such as pirfenidone and nintedanib, significantly reduce the decline of forced vital capacity, these drugs neither promote recovery nor prevent the progression of functional decline and the structural distortion seen in PF. Thus, understanding the cellular and molecular mechanisms in the pathogenesis of IPF may lead to more effective therapies. Recently, several articles have reported new findings on the pathogenesis of PF, which may serve as a potential approach for targeted treatment of PF.
1. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that drives PF
Chemokine (C-C motif) ligand 1 (CCL1) regulates tissue homeostasis through the recruitment of immune cells to sites of injury and inflammatory tissues through its interaction with its receptor. In this way, CCL1 contributes to various human inflammatory diseases. Zhuo-wei Hu team of the Second Xiangya Hospital of Central South University reported that CCL1 was elevated in bronchoalveolar lavage fluid of PF mice and human fibrotic lung tissue[1]. Furthermore, the enhanced expression of CCL1 by immune cells exerted the profibrotic role by activating lung fibroblasts into myofibroblasts via its interaction with autocrine motility factor receptor (AMFR) in fibrotic lung tissues of PF patients and PF mouse models. Mechanistically, CCL1 triggered AMFR phosphorylation that activated E3 ligase activity of AMFR and downstream ubiquitination of the ERK inhibitor Spry1(Fig 1). This relieved the inhibitory effect of Spry1 on ERK-p70S6K signaling activity and enhanced profibrotic protein synthesis. These results present the CCL1-AMFR-ERK signal cascade as a therapeutic target for the treatment of PF.
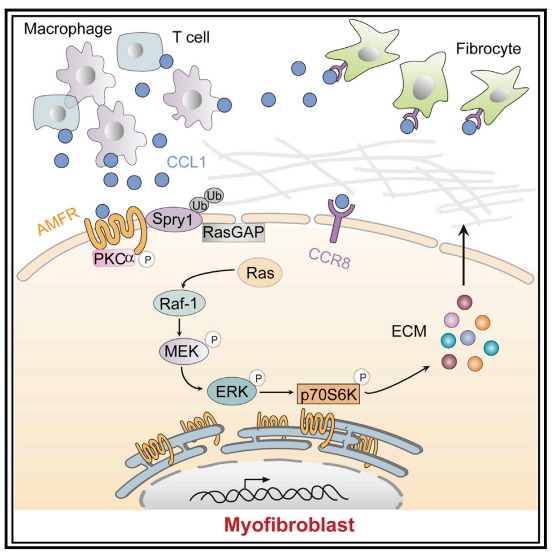
Fig 1. Chemokine CCL1 drives PF through the AMFR-SPRy1 pathway
2. TGFβ2 and TGFβ3 isoforms drive fibrotic disease
Transforming growth factor–β(TGFβ) is a key driver of fibrogenesis. Three TGFβ isoforms (TGFβ1, TGFβ2, and TGFβ3) in mammals have distinct functions in embryonic development; however, the postnatal pathological roles and activation mechanisms of TGFβ2 and TGFβ3 have not been well characterized. Joseph R. Arron team of Genentech Inc proved that the latent forms of TGFβ2 and TGFβ3 can be activated by integrin-independent mechanisms and have lower activation thresholds compared to TGFβ1. Unlike TGFβ1, TGFβ2 and TGFβ3 expression is increased in human lung and liver fibrotic tissues compared to healthy control tissues[2]. Isoform-selective therapeutic inhibition of TGFβ2 and TGFβ3 with a potent allosteric antibody can ameliorate experimental fibrosis in vivo(Fig 2). These findings support isoform-selective TGFβ2 and/or TGFβ3 inhibition as a therapeutic strategy for patients with chronic fibrotic disorders.
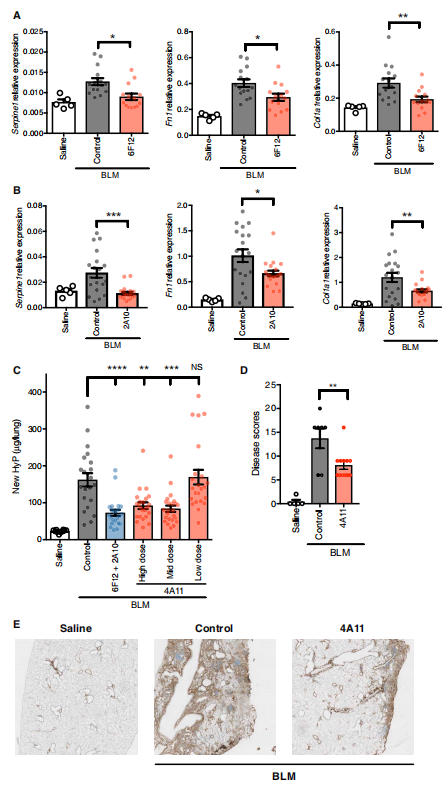
Fig 2. Anti-TGFβ2 and anti-TGFβ3 antibody treatment reduces lung fibrosis in the bleomycin challenge model
3. Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling
The mitochondrial calcium uniporter (MCU) regulates metabolic reprogramming in lung macrophages and the progression of PF. Fibrosis progression is associated with apoptosis resistance in lung macrophages. A. Brent Carter team of University of Alabama at Birmingham found a marked increase in mitochondrial B-cell lymphoma-2 (Bcl-2) in lung macrophages from subjects with idiopathic PF[3]. Whereas Bcl-2 was markedly decreased in mice expressing a dominant-negative mitochondrial calcium uniporter (DN-MCU). MCU regulated metabolic reprogramming to fatty acid β-oxidation, in part, by increasing expression and activity of Cpt1a. Cpt1a, the rate-limiting enzyme for fatty acid β-oxidation, directly interacted with Bcl-2 by binding to its BH3 domain, which anchored Bcl-2 in the mitochondria to attenuate apoptosis. The deletion of Bcl-2 in macrophages protected mice from developing pulmonary fibrosis. Moreover, mice had resolution when Bcl-2 was deleted or was inhibited with ABT-199 after fibrosis was established(Fig 2). These observations implicate an interplay between macrophage fatty acid β-oxidation, apoptosis resistance, and dysregulated fibrotic remodeling.
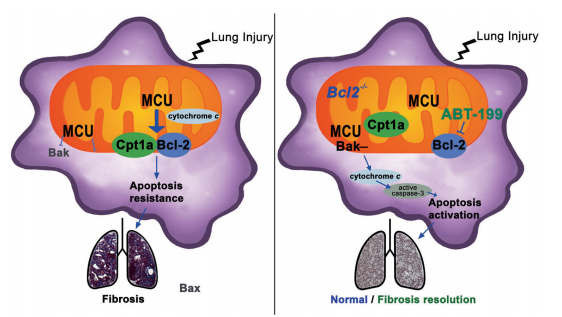
Fig 3. Cpt1a-Bcl-2 binding regulates apoptosis and fibrotic remodeling
Cloud-Clone can not only provide animal models of pulmonary fibrosis, but also cover animal models of other common respiratory diseases (asthma, bronchitis, pulmonary embolism, pneumonia, chronic obstructive pulmonary disease, etc.). It also has various lung disease detection indicators and the proteins involved in the above-mentioned research (CCL1, AMFR, ERK, Spry1, TGFβ2, TGFβ3, Bcl-2, Cpt1a), which can help the majority of scientific researchers in lung disease-related research.
Reference
[1] Liu SS, Liu C, Lv XX, et al. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis[J]. Immunity, 2021, 54(9): 2042-2056.(IF=31.745)
[2] Sun T, Huang Z, Liang WC, et al. TGFβ2 and TGFβ3 isoforms drive fibrotic disease pathogenesis[J]. Sci Transl Med, 2021, 13(605): eabe0407.(IF=17.956)
[3] Gu Linlin,Surolia Ranu,Larson-Casey Jennifer L et al. Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling.[J] .Cell Death Differ, 2021.(IF=15.828)