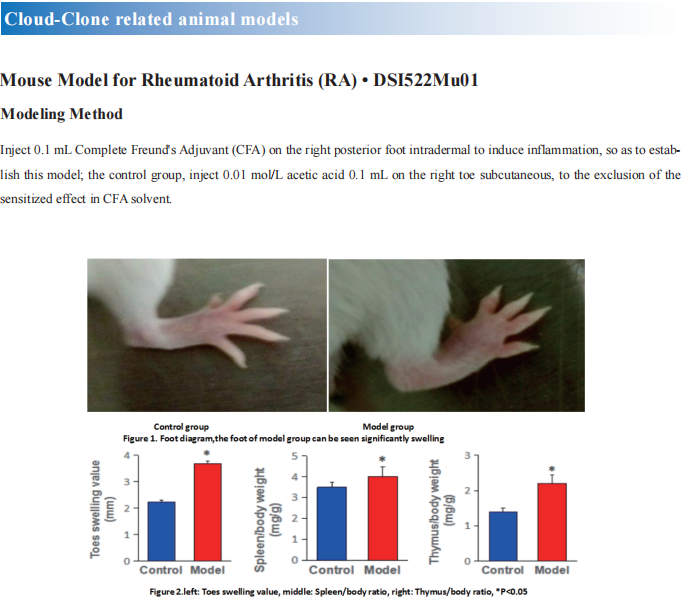
New progress in the study of inflammatory arthritis disease mechanism
Inflammatory arthritis, including spinal arthritis (SpA) and rheumatoid arthritis (RA), are a kind of chronic inflammatory diseases, which will gradually damage the joints of patients and have a negative impact on normal life. SpA is characterized by inflammation in distinct anatomical sites and abnormal new bone formation in the entheses of the spine and peripheral joints; RA is characterized by the production of autoantibodies against extracellular matrix proteins and other targets, synovial inflammation and hyperplasia, and progressive cartilage and bone erosion.
New progress in inflammatory arthritis research
The use of anti-inflammatory drugs, including biologics, may relieve inflammatory arthritis symptoms, but some patients do not respond to any treatment and treatment has potential side effects. Therefore, further advances in understanding the regulatory mechanisms involved in the pathogenesis of inflammatory arthritis may contribute to the development of new therapies with improved treatment outcomes.
1. Macrophage migration inhibitors drive SpA
Macrophage migration inhibitory factor (MIF) is an upstream proinflammatory cytokine that promotes inflammation and influences the differentiation of the adaptive immune response. Studies have shown that MIF concentrations in serum, synovial fluid, or intestinal tissue are higher in SpA patients than in healthy individuals or disease controls, but the pathological role of MIF in SpA remains unclear. Nigil Haroon's team at the Schroeder Institute for Arthritis Research in Canada observed increased expression of MIF and its receptor CD74 in blood and tissue in a mouse model of SpA[1]. MIF overexpression was sufficient to induce SpA-like clinical features in mice with enhanced type 3 immunity, whereas SpA mice treated with a MIF antagonist prevented or attenuated curdlan-induced SpA manifestations(Fig. 1). Mechanistically, MIF intensifies type 3 immunity by boosting human and mouse T regulatory cell (Treg) acquisition of a TH17 cell–like phenotype, including the up-regulation of interleukin-17 (IL-17) and IL-22 in vitro. These results indicate that MIF is a crucial regulator and a potential therapeutic target in type 3 immunity-mediated arthritis.
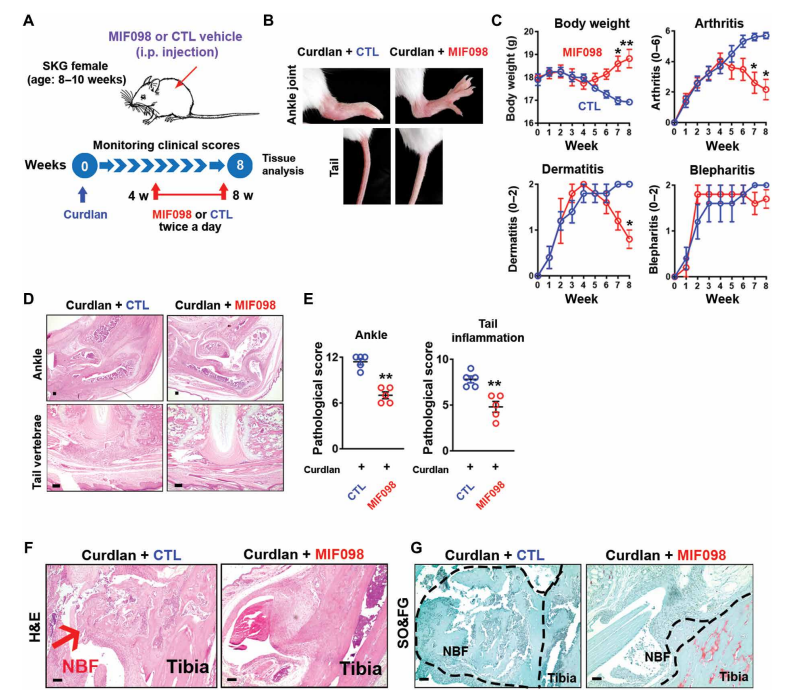
Fig. 1 Therapeutic effects of MIF antagonist (MIF098) on SpA pathologies
2. Del-1 inhibits Tfh cell activation and inflammatory arthritis
Developmental endothelial locus 1 (DEL-1) is secreted by tissue-resident cells (e.g., mesenchymal stromal cells, endothelial cells, and certain macrophage subsets) and regulates both the initiation and resolution of inflammation. Given DEL-1's anti-inflammatory effects, George Hajishengallis and his team at the University of Pennsylvania explored whether DEL-1 could also prevent inflammatory arthritis[2]. By using 2 models of experimental arthritis (collagen-induced arthritis [CIA] and collagen Ab–induced arthritis [CAIA]) and DEL-1 transgenic and KO mice, they showed here that DEL-1 also restrained arthritis. In both models, mice with endothelium-specific overexpression of DEL-1 were protected from arthritis relative to WT controls, whereas arthritis was exacerbated in DEL-1-deficient mice. Mechanistically, DEL-1 inhibited DC-dependent induction of Tfh cells by targeting the LFA-1 integrin on T cells(Fig. 2). Overall, DEL-1 restrained arthritis through a dual mechanism, one acting locally in the joints and associated with the anti-recruitment function of endothelial cell–derived DEL-1; the other mechanism acting systemically in the lymph nodes and associated with the ability of stromal cell–derived DEL-1 to restrain Tfh responses. DEL-1 may therefore be a promising therapeutic for the treatment of inflammatory arthritis.
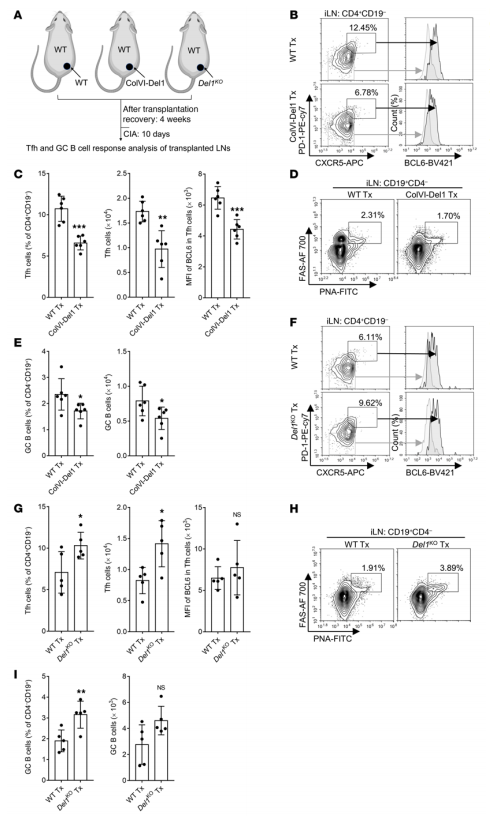
Fig. 2 Lymph node stromal cell–derived DEL-1 inhibits Tfh and GC–B cell responses in vivo
3. Augmenting MNK1/2 activation by c-FMS promotes osteoclastogenesis and arthritic bone erosion
Osteoclasts are bone-resorbing cells that play an essential role in homeostatic bone remodeling and pathological bone erosion. Bone erosion is one of the key clinical features of RA and is closely linked to impaired mobility in patients with RA. Macrophage colony stimulating factor (M-CSF) is abundant in rheumatoid arthritis (RA). However, the role of M-CSF in arthritic bone erosion is not completely understood. The study of Kyung-hyun Park-min team at Rosenwig Genomics Research Center in the United States showed that M-CSF can promote osteoclastogenesis by triggering the proteolysis of c-FMS, a receptor for M-CSF, leading to the generation of FMS intracellular domain (FICD) fragments[3]. Increased levels of FICD fragments positively regulated osteoclastogenesis but had no effect on inflammatory responses. Moreover, myeloid cell-specific FICD expression in mice resulted in significantly increased osteoclast-mediated bone resorption in an inflammatory arthritis model. The FICD formed a complex with DAP5, and the FICD/DAP5 axis promoted osteoclast differentiation by activating the MNK1/2/EIF4E pathway and enhancing NFATc1 protein expression(Fig. 3). These results identified a novel role of c-FMS proteolysis in osteoclastogenesis and the pathogenesis of arthritic bone erosion.
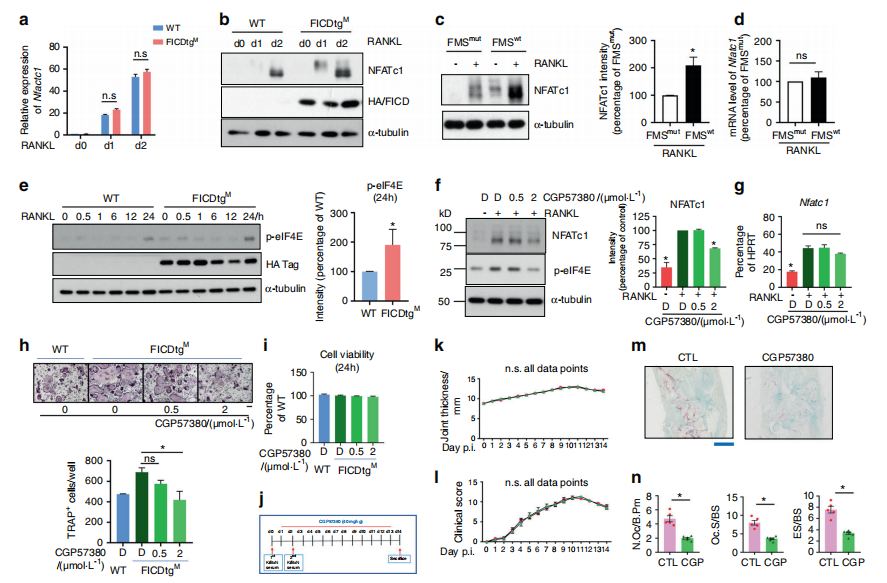
Fig.3 The FICD augments NFATc1 expression by activating the MNK1/2/eIF4E axis
Cloud-clone can not only provide animal models of a variety of bone diseases, including fractures, articular cartilage damage, rheumatoid arthritis, osteoarthritis and other common bone diseases. We also have all kinds of bone metabolism detection indicators and the above MIF, CD74, IL-17, IL-22, M-CSF and other molecular related products, which can help the majority of scientific researchers to carry out research on the treatment of bone diseases.
References
[1]Nakamura A, Zeng F, Nakamura S, et al. Macrophage migration inhibitory factor drives pathology in a mouse model of spondyloarthritis and is associated with human disease[J]. Sci Transl Med. 2021;13(616):eabg1210.(IF=17.956)
[2]Wang H, Li X, Kajikawa T, et al. Stromal cell-derived DEL-1 inhibits Tfh cell activation and inflammatory arthritis[J]. J Clin Invest. 2021;131(19):e150578.(IF=14.808)
[3]Mun SH, Bae S, Zeng S, et al. Augmenting MNK1/2 activation by c-FMS proteolysis promotes osteoclastogenesis and arthritic bone erosion[J]. Bone Res. 2021;9(1):45. (IF=13.567)