New strategies for ameliorating cerebral ischemic injury
Cerebral ischemia refers to the insufficiency of blood supply to the brain due to various reasons. The main etiology is related to cerebral artery stenosis or occlusion, cerebral artery embolism, hemodynamic factors, and hematological factors, and it is one of the main causes of death and disability worldwide.
New strategies for the treatment of cerebral ischemia injury
Although intravascular catheter treatment and thrombolytic therapy have been used widely in some patients, both treatments have limitations. Furthermore, even after successful recanalization, Ischemia enhances inflammation by activating innate and adaptive immune responses, often resulting in brain damage and permanent neurological impairment. Therefore, it is urgent to explore new adjuvant therapy strategies to improve the prognosis of cerebral ischemia.
1. CD300a blockade enhances efferocytosis and ameliorates neuronal deficit after cerebral ischemia
Efferocytosis is the process by which dead cells are engulfed by phagocytes, which helps prevent damage-associated molecular patterns (DAMPs) release and the inflammatory responses that follow. CD300a plays an important role in the regulation of efferocytosis by dephosphorylation of intracellular substrates. Akira Shibuya team of the University of Tsukuba School of Medicine in Japan attempted to target CD300a to understand the role of efferocytosis in the pathology of cerebral ischemia[1]. They showed that mice deficient in CD300a, exhibited ameliorated neurological deficit after middle cerebral artery occlusion (MCAO). CD300a inhibited signaling through the CD300b-DNAX-activation protein 12 (DAP12) signaling pathway to prevent efferocytosis of apoptotic cells. Deficiency of CD300a enhanced efferocytosis by myeloid cells and reduced release of DAMPs from dead cells(Fig.1), resulting in milder inflammation in the penumbral region. These findings reveal an important role of efferocytosis in cerebral ischemia pathology and identified CD300a as a target for immunotherapy in treating cerebral ischemia.
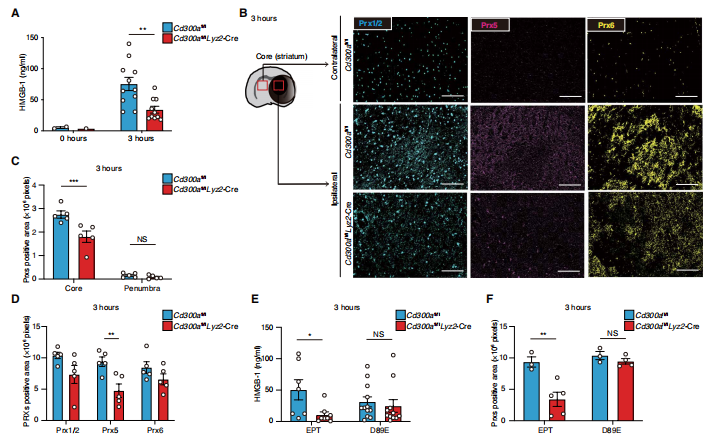
Fig.1 CD300a-deficient mice show decreased DAMPs after ischemic stroke
2. Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment
Ischemia and reperfusion can initiate cascade reactions, such as oxidative stress and inflammation response, which ultimately leads to the irreversible neuron injury. The overproduced reactive oxygen species (ROS) is a major cause of oxidative stress, and it leads to the neuronal necrosis or apoptosis through oxidizing bio-macromolecules and activating apoptotic pathway. Manganese dioxide (MnO2) nanoparticles could consume excess hydrogen peroxide (H2O2) and convert it into O2 in situ. Chen Jiang's team, School of Pharmacy, Fudan University, developed a macrophage-disguised honeycomb MnO2 nanosphere loaded with fingolimod to salvage the ischemic penumbra[2]. The nanoparticles can accumulate actively in the damaged brain viamacrophage-membrane protein-mediated recognition with cell adhesion molecules that are overexpressed on the damaged vascular endothelium, and can be decomposed in acidiclysosome for cargo release, so as to reduce oxidative stress and eventually reversing the proinflammatory microenvironment and reinforcing the survival of damaged neuron(Fig.2). This biomimetic nanomedicine raises new strategy for multitargeted combined treatment of cerebral ischemia.
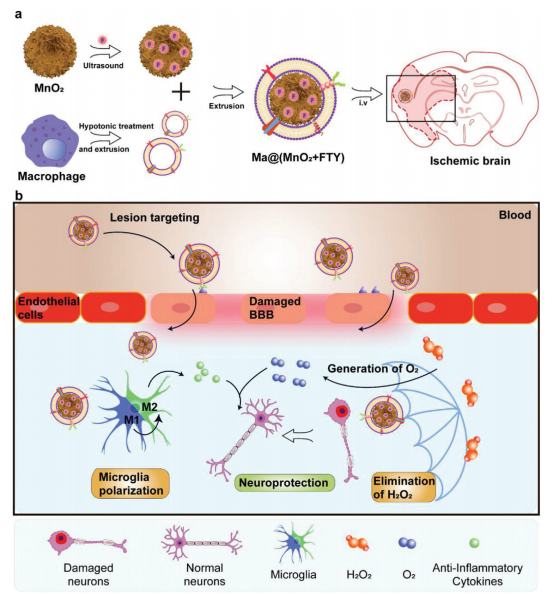
Fig.2 MnO2 nanoparticles promote neuronal formation and repair in ischemic brain injury
3. PKM2 promotes neutrophil activation and cerebral ischemia inflammation
Pyruvate kinase muscle 2 (PKM2) has been implicated not only as a critical regulator of aerobic glycolysis but also as an activator of transcription of pro-inflammatory mediators, including IL-1 and IL-6. Anil Chauhan and his colleagues at the University of Iowa explored the role of PKM2 in the pathogenesis of cerebral ischemia[3]. Genetic deletion of PKM2 in myeloid cells limited inflammatory response in peripheral neutrophils following cerebral ischemia/reperfusion. In the cerebral ischemia models, deletion of PKM2 in myeloid cells either in wild-type or hyperlipidemic mice reduced infarcts and enhanced long-term sensorimotor recovery. Regional cerebral blood flow improved in bone marrow cell-specific PKM2 deficient mice, accompanied by reduced cerebral thrombotic inflammation after ischemia. Mechanistically, PKM2 regulates post-ischemic inflammation in peripheral neutrophils by promoting STAT3 phosphorylation(Fig.3). These findings identify PKM2 as a novel therapeutic target to improve brain injury after reperfusion.
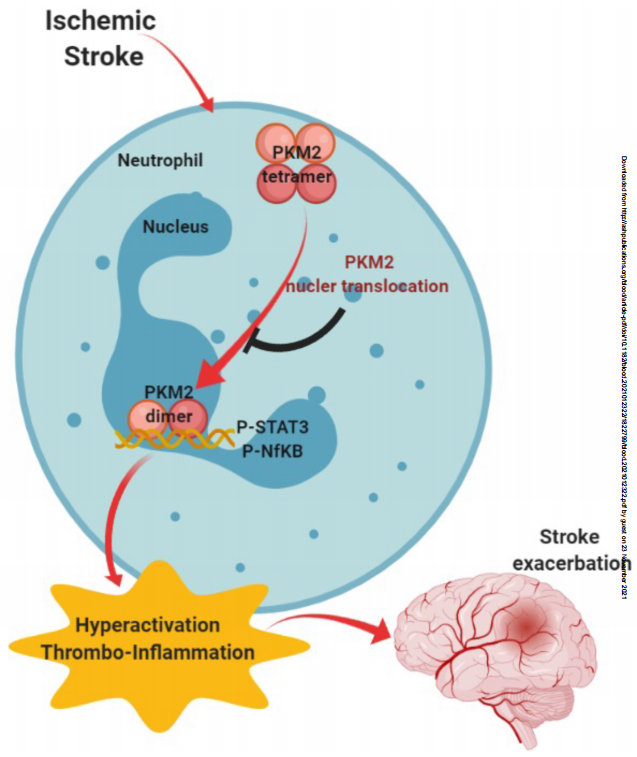
Fig.3 PKM2 promotes neutrophil activation and cerebral ischemia inflammation
4. Smoothened reduces glutamate toxicity to improve cerebral ischemic injury
In ischemia, large amounts of released glutamate quickly accumulate in the extracellular space largely due to insufficient reuptake, triggers a series of reactions such as periinfarct depolarization, inflammation, and programmed cell death, suggesting that glutamate toxicity likely plays a prominent role in the first phases after ischemic brain damage. Smoothened (SMO) is the key mediator of sonic hedgehog (SHH), while SHH signal plays a key role in brain tissue repair during post-ischemia reperfusion. Recently, Yizheng Wang and his team at the Center for Brain Science, Beijing Institute of Basic Medical Sciences, reported that activation of SMO inhibited GLT-1 activities, leading to increased extracellular glutamate[4]. Inhibiting SMO, maintained GLT-1 membrane expression, lowered extracellular glutamate, reduced infarct volume, and improved neurological functions in mice(Fig.4). They further applied SMO antagonists to cynomolgus monkeys and found that the treatment had both short- and long- term protective effects in ischemic models. Thus, SMO represents a potential therapeutic target to protect the brain from ischemic damage.
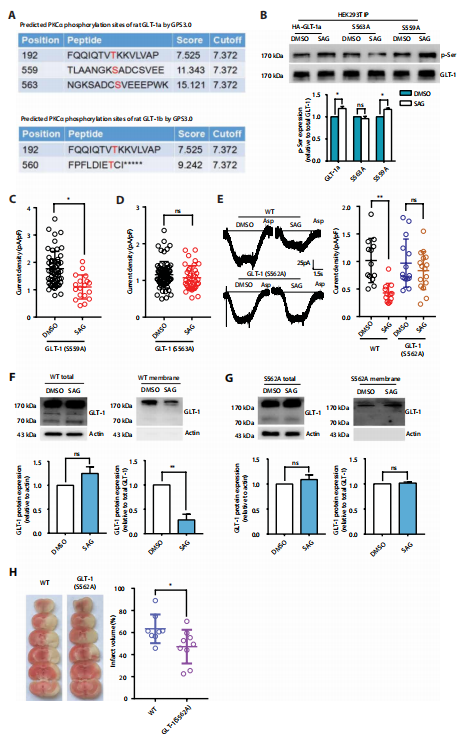
Fig.4 Inhibiting SMO-induced GLT-1a phosphorylation maintains its activity
References
[1] Nakahashi-Oda C, Fujiyama S, Nakazawa Y, et al. CD300a blockade enhances efferocytosis by infiltrating myeloid cells and ameliorates neuronal deficit after ischemic stroke[J]. Sci Immunol. 2021, 6(64):eabe7915.(IF=17.727)
[2] Li C, Zhao Z, Luo Y, et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke[J]. Adv Sci (Weinh). 2021, 8(20):e2101526.(IF=16.806)
[3] Dhanesha N, Patel RB, Doddapattar P, et al. PKM2 promotes neutrophil activation and cerebral thrombo-inflammation: Therapeutic implications for ischemic stroke [J]. Blood. 2021, blood.2021012322.(IF=22.113)
[4] Wang Y, Lu S, Chen Y, et al. Smoothened is a therapeutic target for reducing glutamate toxicity in ischemic stroke[J]. Sci Transl Med. 2021, 13(610):eaba3444.(IF=17.956)
Cloud-Clone not only provides animal models of cerebral ischemia, but also has various commonly used cerebrovascular disease indicators and the above-mentioned CD300a, PKM2 and SMO protein related products, which can help the majority of scientific researchers to carry out researches on the treatment of cerebral ischemia.

