Precise Correction of Lhcgr Mutation in Stem Leydig Cells by Prime Editing Rescues Hereditary Primary Hypogonadism in Mice
Leydig cells (LCs), which are located in the interstitial compartment of the testes and nestled among the seminiferous tubules, are primarily responsible for the production of testosterone. Thus, LCs are indispensable for the development and maintenance of the masculine phenotype, endocrine homeostasis, and reproductive function. Hereditary primary hypogonadism (HPH) is caused by malfunction at the level of the testes due to genetic causes which impair LCs function. This condition is characterized by low or absent testosterone levels and high gonadotropins levels, underdeveloped masculine phenotype, and severely impaired spermatogenesis. Luteinizing hormone/choriogonadotropin receptor (LHCGR) plays a vital role in LCs differentiation and testosterone synthesis. The mutation of Lhcgr causes testosterone deficiency and further contributes to impaired sexual development, arrested spermatogenesis and infertility, thus serves as prototype of HPH. Testosterone replacement therapy (TRT) is the first choice for treating HPH, as exogenous TRT can largely reverse low serum levels of testosterone and partially ameliorate hypogonadism-associated symptoms. However, exogenous TRT induces many adverse effects, such as sleep apnoea, stroke, heart attack, and prostate tumorigenesis. Notably, exogenous testosterone has yet to mimic the physiological patterns of testosterone secretion and aggravates spermatogenesis failure. Therefore, demands for alternative therapies to TRT are noticeably increasing.

On September 11, 2023, Andy Peng Xiang team of Zhongshan School of Medicine of Sun Yat-sen University and Chunhua Deng team of the First Affiliated Hospital of Sun Yat-sen University published a paper titled “Precise Correction of Lhcgr Mutation in Stem Leydig Cells by Prime Editing Rescues Hereditary Primary Hypogonadism in Mice” in Advanced Science. The results suggest that prime editing-based gene editing in stem Leydig cells (SLCs) ex vivo is a promising strategy for hereditary primary hypogonadism (HPH) therapy and is potentially leveraged to address more hereditary diseases in reproductive system.

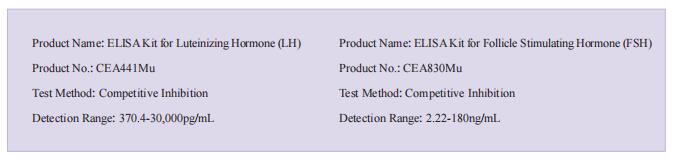
The kits [ELISA Kit for Luteinizing Hormone (LH), CEA441Mu; ELISA Kit for Follicle Stimulating Hormone (FSH), CEA830Mu] of Cloud-Clone brand were chosed in this article, we are so proud for supporting the reaserchers.
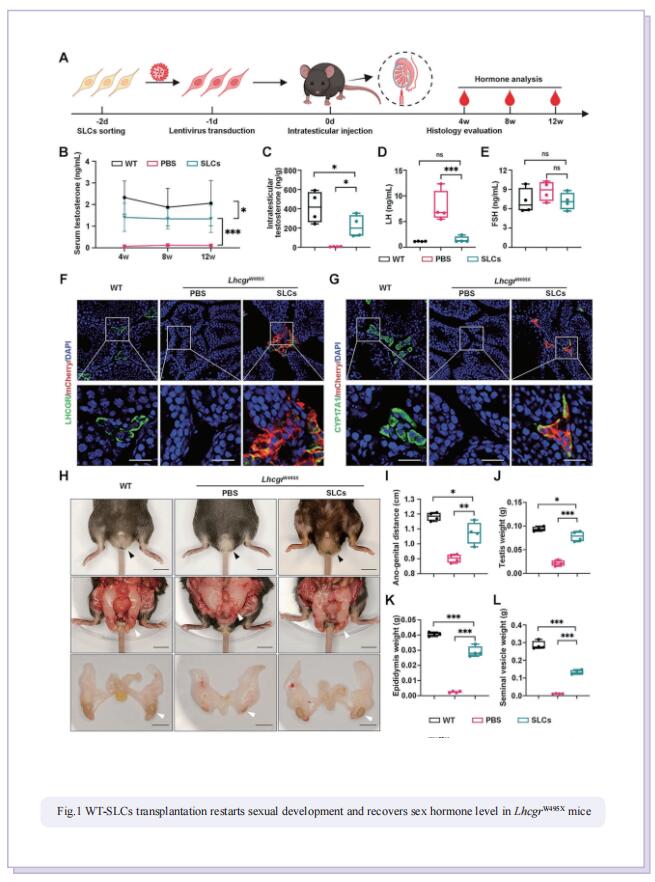
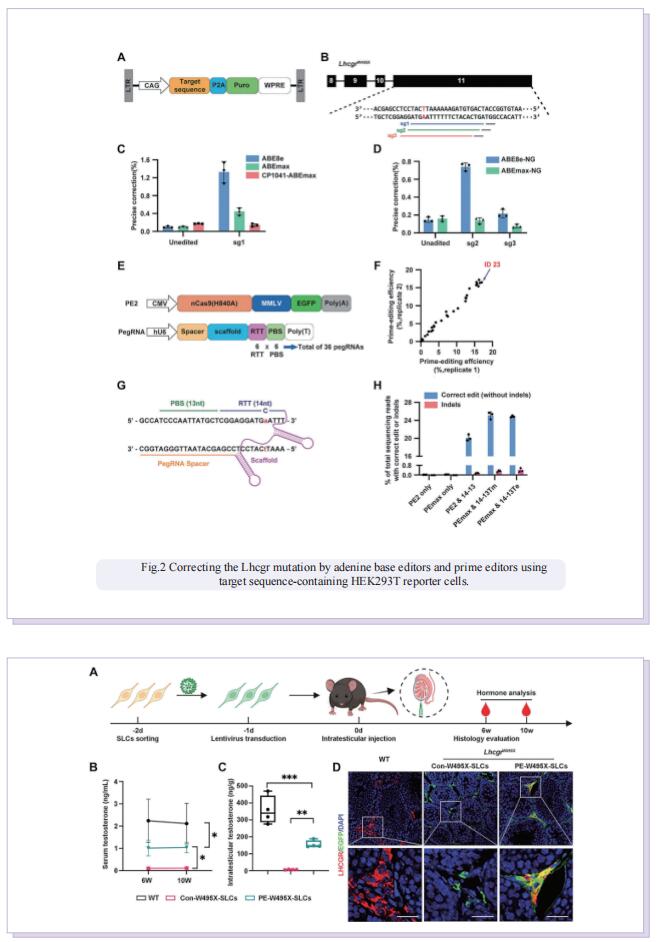
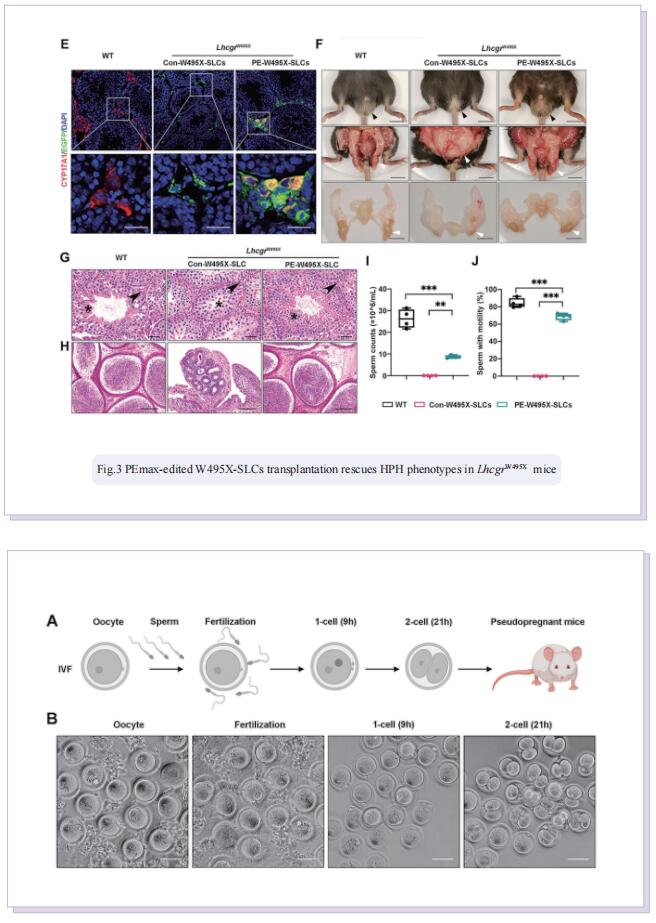
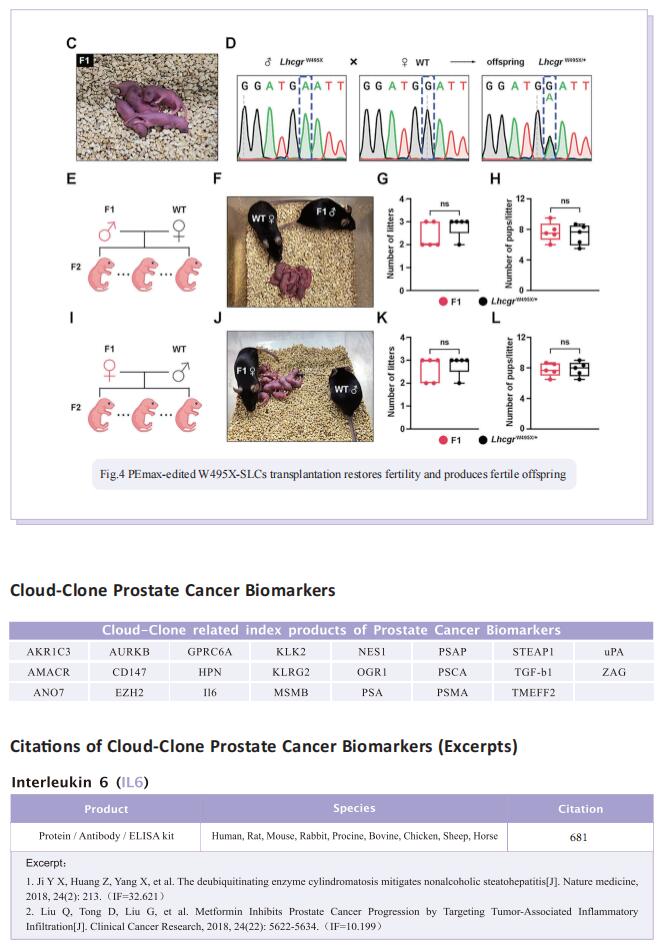
Stem Leydig cells (SLCs), which are capable of regenerating new LCs through proliferation and differentiation, play a critical role in maintaining LCs homoeostasis in adult testes. SLCs transplantation is a promising therapy for male primary hypogonadism. However, SLCs transplantation can’t be achieved due to the hereditary gene defect of SLCs from HPH patients. The large-scale applications of gene-edited stem cell-derived cells in preclinical and clinical studies suggest that once hypogonadism-inducing genetic mutations have been corrected in SLCs, they should be suitable for autologous transplantation to rescue HPH by differentiating into functional LCs. Here, the authors first generated an LhcgrW495X mutant mouse model based on a nonsense point mutation of Lhcgr that is observed in HPH patients. To verify the efficacy and feasibility of SLCs transplantation in treating HPH, wild-type SLCs are transplanted into LhcgrW495X mice, in which SLCs obviously rescue HPH phenotypes. Through comparing several editing strategies, optimized PE2 protein (PEmax) system is identified as an efficient and precise approach to correct the pathogenic point mutation in Lhcgr. Furthermore, delivering intein-split PEmax system via lentivirus successfully corrects the mutation in SLCs from LhcgrW495X mice ex vivo. Gene-corrected SLCs from LhcgrW495X mice exert ability to differentiate into functional Leydig cells in vitro. Notably, the transplantation of gene-corrected SLCs effectively regenerates Leydig cells, recovers testosterone production, restarts sexual development, rescues spermatogenesis, and produces fertile offspring in LhcgrW495X mice.
In summary, these results suggest that PE-based therapeutic editing in SLCs ex vivo is a promising strategy for HPH. It is expected that the proof-of-concept data will set the stage for further studies and open a new avenue for treating not only HPH but other genetic diseases that affect the reproductive function.