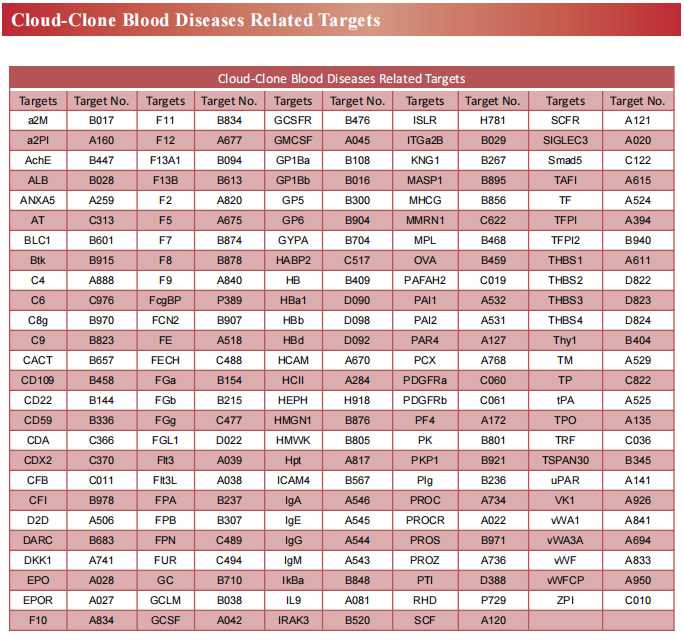
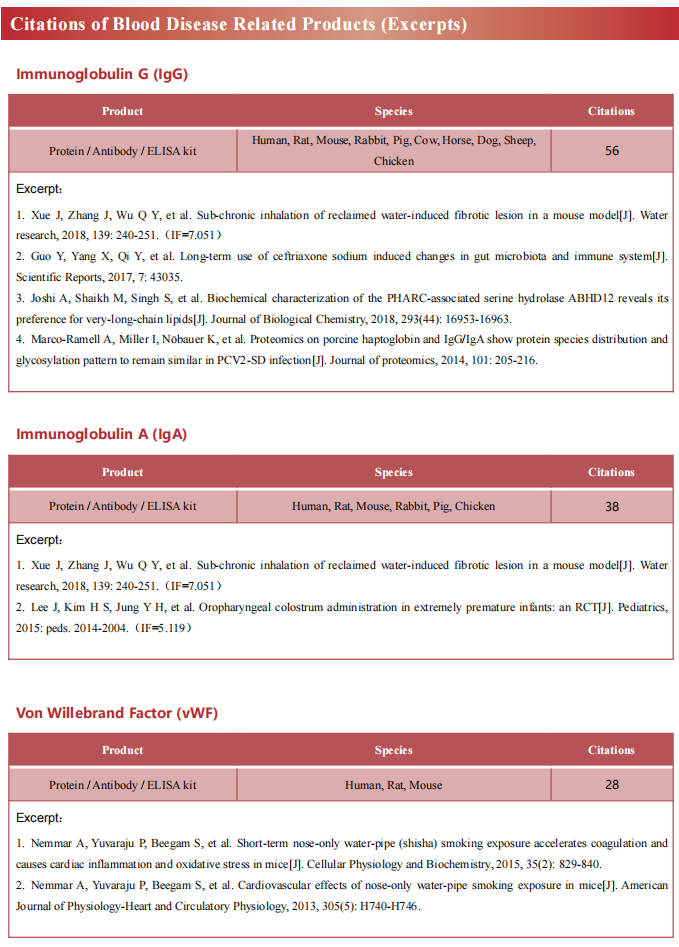
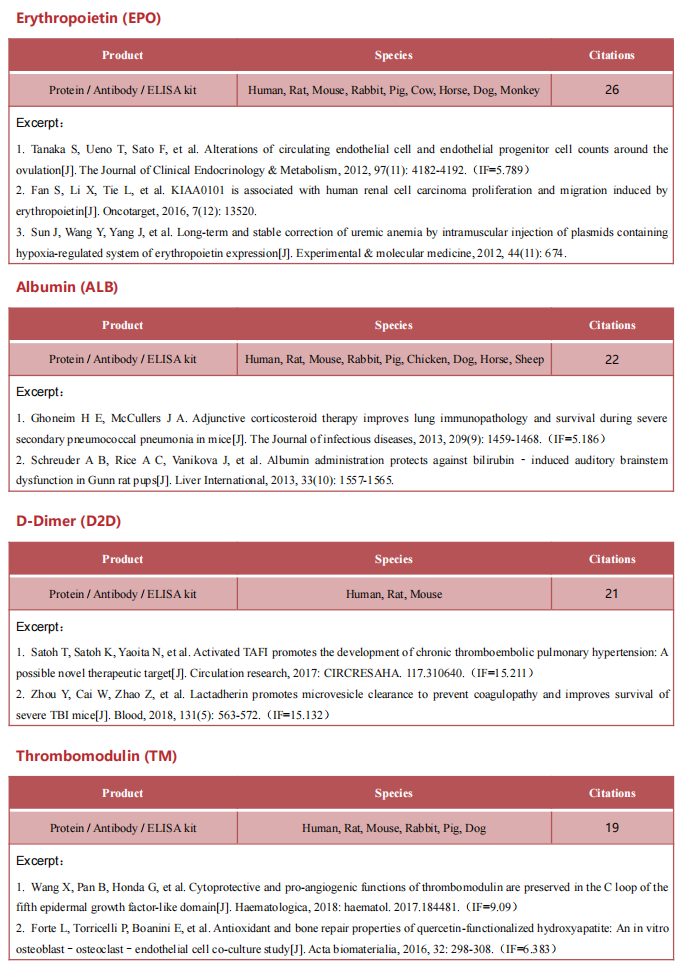
Recent advances in the mechanism of acute myeloid leukemia
Acute myeloid leukemia (AML) is a fatal form of hematopoietic malignancy by abnormal hematopoietic maturation, uncontrolled cellular proliferation,with 5-year survival rate of adult patients only 24%.Chemotherapy is standard-of-care, but therapeutic regimens have not changed dramatically in several decades and are often poorly tolerated. Despite the progress of therapeutic approaches such as allogeneic hematopoietic stem cell transplantation (allo-HSCT) and chimeric antigen receptor (CAR) T cell treatment, the survival of AML patients has not been significantly improved. Novel treatments for AML are urgently needed. Therefore, further understanding of the pathogenesis of AML may contribute to the development of new therapies to improve treatment outcomes.
1. NEAT1 Suppresses AML Stem Cell Self-Renewal and Leukemogenesis through Inactivation of Wnt Signaling
As an essential component of paraspeckles, nuclear paraspeckle assembly transcript 1 (NEAT1) localizes in the nucleus, promoting progression of various malignant solid tumors. Pengcheng Bu, College of Life Sciences, University of Chinese Academy of Sciences, and his team showed NEAT1_1 is downregulated in leukemia stem cells (LSCs) and its decreased expression correlates with recurrence in AML patients[1]. NEAT1_1 suppresses leukemogenesis and LSC function, thus inhibiting the development of AML. Mechanistically, NEAT1_1 is released from the nucleus into the cytoplasm of AML cells, regulated by transcription factor C/EBPβ and nuclear protein NAP1L1. Cytoplasmic NEAT1_1 interacts with Wnt component DVL2 and E3 ubiquitin ligase Trim56, facilitates Trim56-mediated DVL2 degradation, and thus suppresses Wnt signaling(Fig.1). Collectively, the findings show NEAT1_1 is translocated from the nucleus to the cytoplasm and acts as a tumor suppressor in AML.
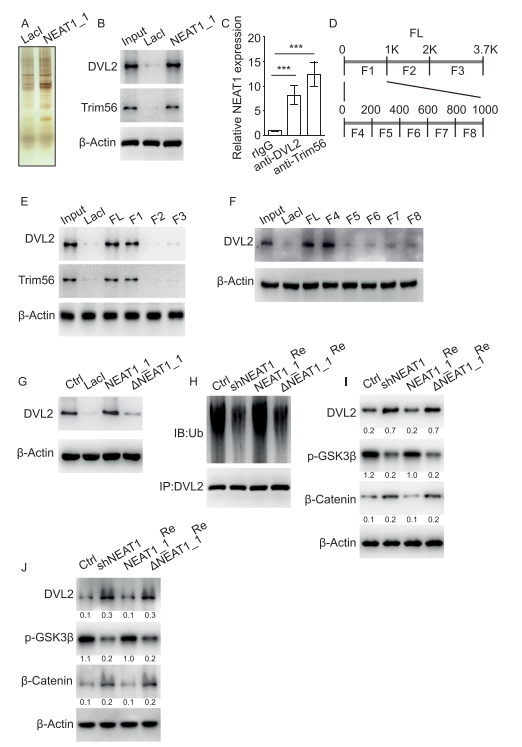
Fig.1 NEAT1_1 suppresses Wnt signaling by interaction with DVL2 and Trim56
2. KAT6A and ENL form an epigenetic transcriptional control module to drive critical leukemogenic gene expression programs
Differentiation therapy provides an alternative in which self-renewal is depleted by inducing myeloid maturation programs, thereby extinguishing proliferation. This approach is curative in the promyelocytic AML subtype (APL) and promising in IDH-mutant AMLs, but has not been successful in other subtypes. To expand the applicability of differentiation therapy, it is critical to understand the mechanisms regulating AML cell fate decisions. Epigenetic regulators are of marked importance for sustaining the transcriptional programs that drive AML differentiation arrest. M. Andrés Blanco, School of Veterinary Medicine, University of Pennsylvania, and his team designed a positive selection gain-of-differentiation CRISPR screen to identify chromatin regulators whose inhibition induces therapeutic differentiation of AML cells[2]. This screen identified the histone acetyltransferase KAT6A as a novel regulator of myeloid differentiation that drives critical leukemogenic gene expression programs. Through a combination of experimental and bioinformatic approaches, they showed that KAT6A collaborates with ENL to drive a MYC-centric gene expression program by providing promoter H3K9ac for ENL binding. Inhibition of KAT6A has strong anti-AML phenotypes in vitro and in vivo(Fig.2), suggesting that KAT6A small molecule inhibitors could be of high therapeutic interest for mono or combinatorial differentiation-based treatment of AML.
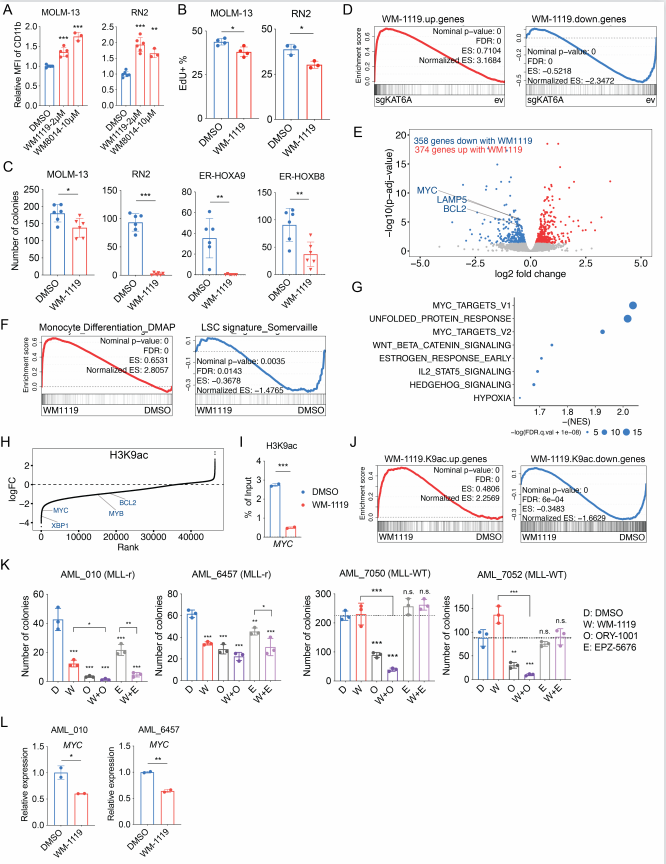
Fig.2 Treatment with WM-1119 inhibits KAT6A-mediated transcriptional program and AML growth
3. PP2A is a therapeutically targetable driver of cell fate decisions via a c-Myc/p21 axis in Acute Myeloid Leukemia
Aberrant protein phosphorylation is one of the leading mechanisms by which malignant cells evade cell death and differentiation. Protein phosphatase 2A (PP2A) has been shown to be a tumor suppressor in a variety of cancers and is usually inactivated in myeloid leukemia. Natarajan Muthusamy of the Ohio State University and his team identified that the phosphatase silencing of PP2A directly contributes to differentiation block in AML[3]. Gene expression and mass cytometric profiling reveal that PP2A activation modulates cell cycle and transcriptional regulators that program terminal myeloid differentiation. Using a novel pharmacological agent OSU-2S in parallel with genetic approaches, they discovered that PP2A enforces c-Myc and p21 dependent terminal differentiation, proliferation arrest and apoptosis in AML. PP2A activation decreases leukemia initiating stem cells, increases leukemic blast maturation, and improves overall survival in murine AML models in-vivo(Fig.3). These findings identify the PP2A/c-Myc/p21 axis as a critical regulator of the differentiation/proliferation switch in AML that can be therapeutically targeted in malignancies with dysregulated maturation fate.
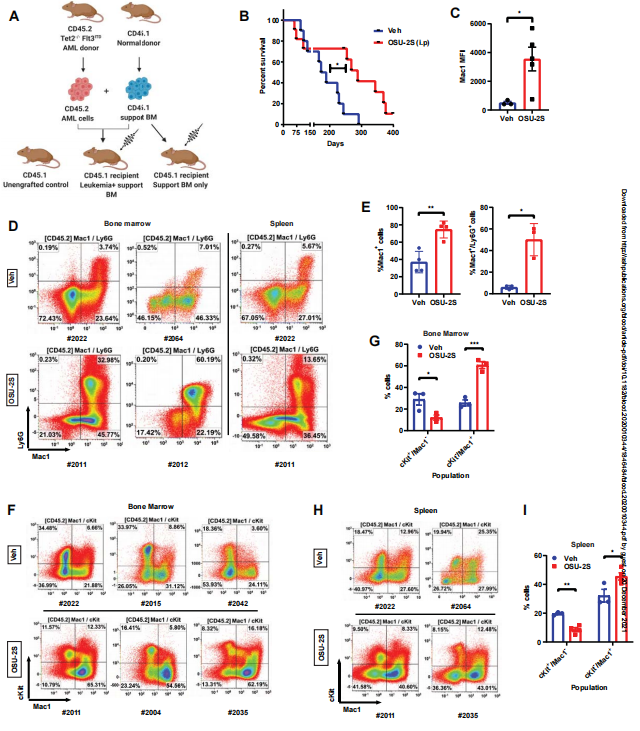
Fig.3 OSU-2S induces maturation and prolongs survival in murine AML model
References
[1]Yan H, Wang Z, Sun Y, Hu L, Bu P. Cytoplasmic NEAT1 Suppresses AML Stem Cell Self-Renewal and Leukemogenesis through Inactivation of Wnt Signaling [J]. Adv Sci (Weinh). 2021, 8(22):e2100914.(IF=16.806)
[2]Yan F, Li J, Milosevic J, et al. KAT6A and ENL form an epigenetic transcriptional control module to drive critical leukemogenic gene expression programs [J]. Cancer Discov. 2021, candisc.1459.2020.(IF=39.397)
[3]Goswami S, Mani R, Nunes J, et al. PP2A is a therapeutically targetable driver of cell fate decisions via a c-Myc/p21 axis in Acute Myeloid Leukemia [J]. Blood. 2021, blood.2020010344.(IF=22.113)
Cloud-Clone not only provides leukemia detection related indicator products, but also has the above-mentioned C/EBPβ, NAP1L1, PP2A protein and Wnt, MYC pathway related indicator products, which can help the general scientific research workers to conduct leukemia related research.