Targeted antitumor immunity improves the efficacy of tumor therapy
Tumor immunity is a process of immune response to the occurrence and development of tumors based on tumor antigenicity and immune function of the body. Antitumor immunotherapy is to stimulate and enhance the immune function of the body in order to control and kill tumor cells.
Antitumor immunity improves the therapeutic effect of tumor
Radiation therapy and chemotherapy are conventional treatments for cancer that have long been in use, but neither of these treatments can completely destroy tumor cells. The last surviving tumor cells need to be taken care of by the body's immune system. Therefore, it is of great significance to explore the mechanism of anti-tumor immunity in improving tumor therapy.
1. ZBP1-MLKL signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation
Necroptosis, a form of regulated necrosis, participates in tumor development and dying cell immunogenicity. Liufu Deng, Shanghai Institute of Immunology, School of Medicine, Shanghai Jiao Tong University, and team found that the ZBP1-MLKL necroptotic cascade in irradiated tumor cells was essential for antitumor immunity [1]. Mechanistically, the ZBP1-MLKL necroptotic cascade induced cytoplasmic DNA accumulation in irradiated tumor cells and, in turn, autonomously activated cGAS-STING signaling, thus creating a positive feedback loop between those two pathways to drive persistent inflammation(Fig.1). Accordingly, ablation of caspase-8 enhanced STING pathway activation and the antitumor effects of radiation by activating MLKL. These findings reveal that ZBP1-MLKL necroptosis signaling maximized radiation-induced antitumor immunity through mutual interaction with the tumor cell-intrinsic STING pathway.
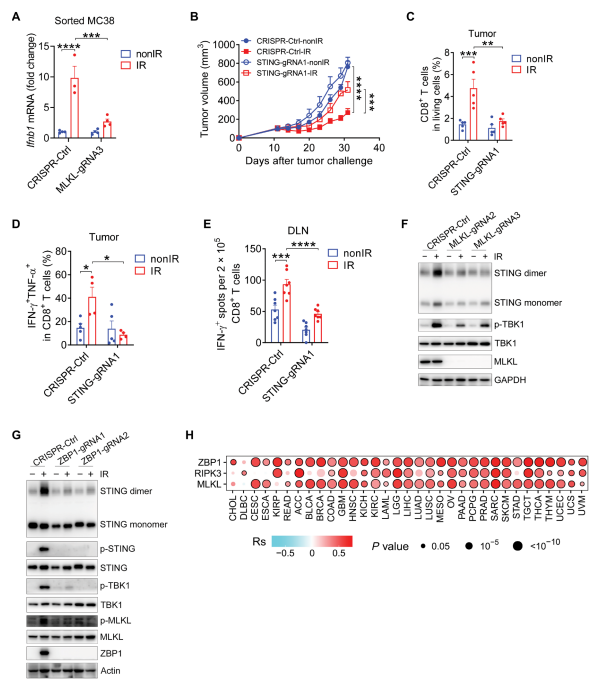
Fig.1 The ZBP1-MLKL necroptotic cascade promotes the activation of STING pathway in tumors after radiation treatment
2. Microenvironment IL-6 inhibits the anticancer immune response produced by cytotoxic chemotherapy
Cytotoxic chemotherapeutics primarily function through DNA damage-induced tumor cell apoptosis, although the inflammation provoked by these agents can stimulate anti-cancer immune responses. The study of Michael T. Hemann and his team at the David H. Koch Institute for Comprehensive Cancer showed that chemotherapy-induced anti-cancer immunity is suppressed by the tumor microenvironment through production of the cytokine IL-6[2]. The chemotherapeutic doxorubicin is curative in IL-6-deficient mice through the induction of CD8+ T-cell-mediated anti-cancer responses(Fig.2), while moderately extending lifespan in wild type tumor-bearing mice. The results suggest that IL-6 is a key regulator of anti-cancer immune responses induced by genotoxic stress and that its inhibition can switch cancer cell clearance from primarily apoptotic to immunogenic, promoting and maintaining durable anti-tumor immune responses.
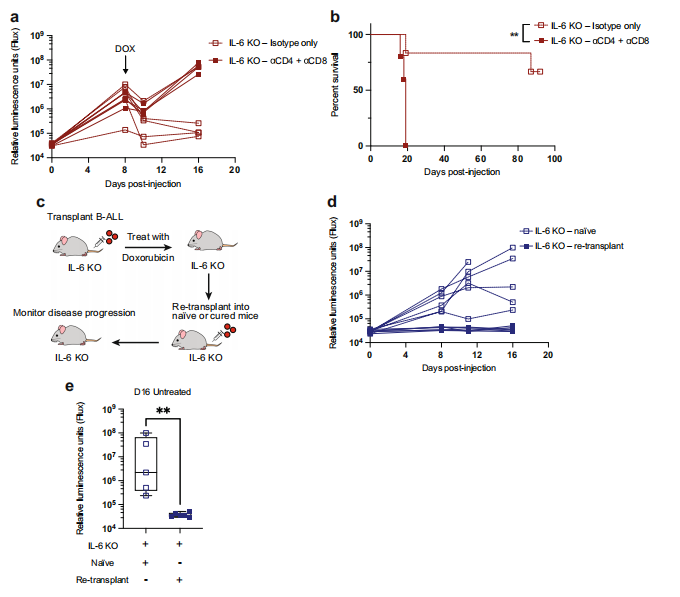
Fig.2 T-cell-dependent anti-tumor immunity develops after doxorubicin treatment of IL-6 KO leukemic mice
3. Loss of tissue transglutaminase boosts antitumor T cell immunity by altering STAT1/STAT3 phosphorylation
Tissue transglutaminase (TG2), an enzyme overexpressed in cancer cells, promotes metastasis and resistance to chemotherapy. Daniela Matei, Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine and his team observed that host TG2 loss delayed tumor growth and ascites accumulation and caused increased infiltration of CD8+ T cells and decreased numbers of myeloid cells in the peritoneal fluid by using a TG2-/- syngeneic ovarian cancer mouse model [3]. Mechanistically, absence of TG2 augmented signals promoting T cell activation, such as increased cytokine-induced STAT1 and attenuated STAT3 phosphorylation in T cells(Fig.3). In response to the more robust immune response caused by loss of TG2, cancer cells growing intraperitoneally exhibited an interferon-γ(IFN-γ) responsive gene signature and underwent apoptosis. Those results prompt TG2 acts as an attenuator of antitumor T cell immunity and is a new immunomodulatory target.
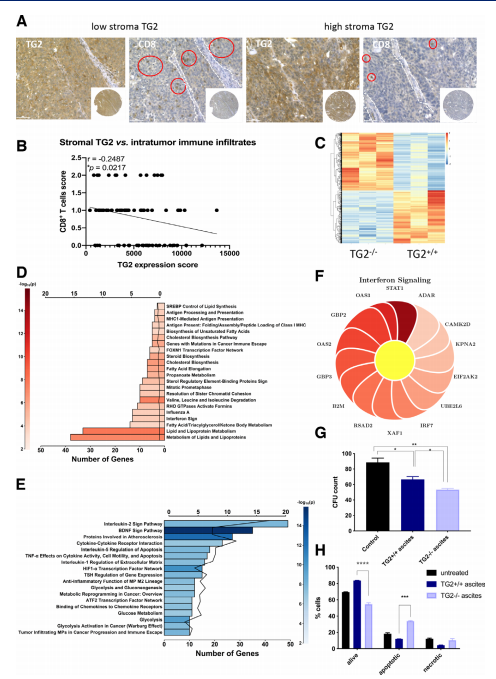
Fig.3 Effects of TG2 expressed in the tumor microenvironment on tumor cells
4. GABA elicits IL-10+ macrophages to limit anti-tumour immunity
Lymphocytes are regulated v a variety of receptor interactions with soluble and cell-bound proteins. Sidonia Fagarasan and his team at the Riken Center for Integrative Medicine in Yokohama, Japan showed that B cell-derived GABA promotes monocyte diferentiation into anti-infammatory macrophages that secrete interleukin-10 and inhibit CD8+ T cell killer function(Fig.4)[4]. In mice, B cell defciency or B cell-specifc inactivation of the GABA-generating enzyme GAD67 enhances anti-tumour responses. The study reveals that, in addition to cytokines and membrane proteins, small metabolites derived from B-lineage cells have immunoregulatory functions, which may be pharmaceutical targets allowing fne-tuning of immune responses.
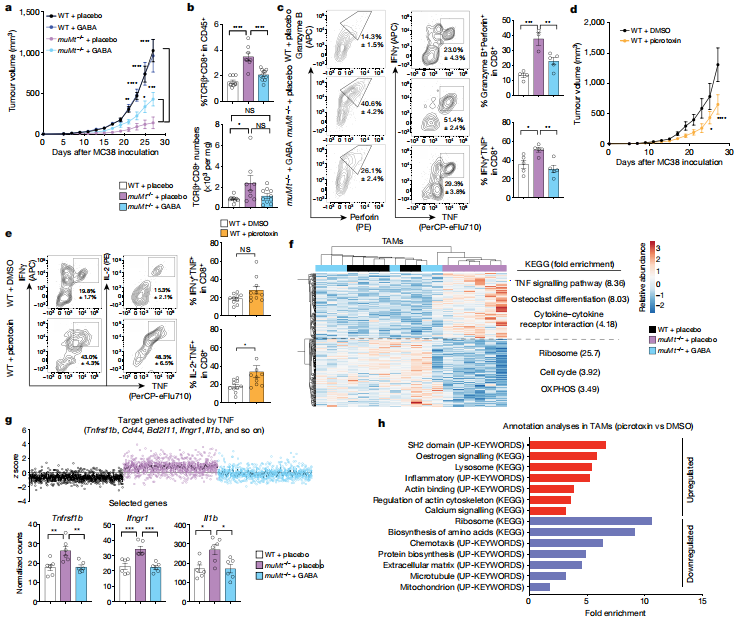
Fig.4 B cells limit anti-tumour responses via GABA
Cloud-Clone provide a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. It also has products related to various cancer detection indicators, cytokines, apoptosis pathways and STING pathways, which can help the majority of researchers to carry out cancer immunity-related research.
References
[1]Yang Y, Wu M, Cao D, et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation[J]. Sci Adv. 2021, 7(41):eabf6290.(IF=14.136)
[2]Bent EH, Millán-Barea LR, Zhuang I, et al. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy[J]. Nat Commun. 2021, 12(1):6218. (IF=14.919)
[3]Sima LE, Chen S, Cardenas H, et al. Loss of host tissue transglutaminase boosts antitumor T cell immunity by altering STAT1/STAT3 phosphorylation in ovarian cancer[J]. J Immunother Cancer. 2021, 9(9):e002682.(IF=13.751)
[4]Zhang B, Vogelzang A, Miyajima M, et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity [J]. Nature. 2021;10.1038/s41586-021-04082-1.(IF=49.962)

