Targeting cardiac hypertrophy in the treatment of heart failure
Myocardial hypertrophy is a slowly developing compensatory mechanism of cardiac function, which mainly occurs in the case of long-term stress overload. It is manifested as an increase in the total amount of cardiac muscle and a strengthening of its contractility, so that the heart can maintain normal blood circulation. However, this compensatory function also has its disadvantages, mainly because hypertrophy myocardial oxygen demand increases, and coronary artery blood supply often can not be satisfied, resulting in myocardial ischemia, which will eventually lead to the decrease of myocardial contractility.
Targeting myocardial hypertrophy in the treatment of heart failure
Heart failure (HF) is the terminal stage of most cardiovascular diseases with high morbidity and mortality. Pathological myocardial hypertrophy is the main independent risk factor for HF development. On the contrary, exercise-induced physiological hypertrophy is beneficial to the prevention of HF. Therefore, exploring the mechanism of myocardial hypertrophy may help prevent and ameliorate HF.
Pathological myocardial hypertrophy and HF
In the case of pressure overload, resulting from aortic stenosis or systemic arterial hypertension, the heart often develops an adaptive hypertrophy, and eventually to systolic heart failure, if the stress is sustained. Suppressing myocardial hypertrophy in patients with heart failure enhances contractile function and improves clinical prognosis. Zhiwei Yang, Beijing Engineering Research Center for Experimental Animal Models of Human Critical Diseases, and his team found that in mice, the dopamine D5 receptor (D5R) expression in the left ventricle (LV) progressively decreases with worsening of transverse aortic constriction-induced left ventricular hypertrophy[1]. These findings indicated delivery of cardiac-specific D5R may be a novel and effective gene therapeutic strategy for improving cardiac function. Then, a reversible treatment of left ventricular hypertrophy with Drd5 nucleic acids delivered by tobramycin-based hyperbranched polyaminoglycoside (SS-HPT) is studied. The heart-specific increase in D5R expression by SS-HPT/Drd5 plasmid in the early stage of left ventricular hypertrophy attenuates myocardial hypertrophy and fibrosis by preventing oxidative and endoplasmic reticulum (ER) stress and ameliorating autophagic dysregulation(Fig.1). By contrast, SS-HPT/Drd5 siRNA promotes the progression of left ventricular hypertrophy and accelerates the deterioration of myocardial function into heart failure. The results show a cardiac-specific beneficial effect of SS-HPT/Drd5 plasmid on myocardial remodeling and dysfunction, which may provide an effective therapy of patients with left ventricular hypertrophy and heart failure.
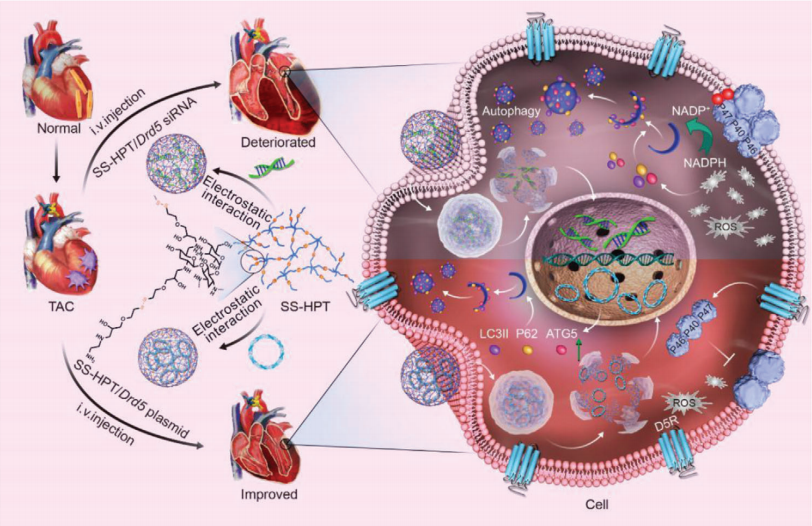
Fig.1 Illustration of the mechanism of the deterioration and improvement of left ventricular hypertrophy, mediated by functional polyaminoglycoside vectors (SS-HPT) loaded with Drd5 siRNA (SS-HPT/Drd5 siRNA) or Drd5 plasmid (SS-HPT/Drd5 plasmid)
Physiological myocardial hypertrophy and HF
The protective effects of exercise training on the cardiovascular system have been widely recognized. Yulin Liao, Department of Cardiology, Nanfang Hospital, Southern Medical University, and his team found that after transverse aortic constriction, the EHP group (submitted to 21 days of swimming training to develop physiological myocardial hypertrophy) showed less increase in myocardial hypertrophy, pulmonary congestion, smaller left ventricular dimensions and end-diastolic pressure, and a larger left ventricular ejection fraction and maximum pressure change rate than sedentary mice[2]. Quantitative polymerase chain reaction revealed that the long noncoding myosin heavy chain–associated RNA transcript Mhrt779 was one of the markedly upregulated lncRNAs in the EHP group. Silencing of Mhrt779 attenuated the antihypertrophic effect of EHP in mice with transverse aortic constriction and in cultured cardiomyocytes treated with angiotensin II, and overexpression enhanced the antihypertrophic effect. Mechanism analysis showed that Mhrt779 inhibited the activation of the pressure-overload induced Hdac2/ P-Akt/P-GSK3 β pathway and increased resistance to pathological stress (Fig.2). In addition, Junjie Xiao, Institute of Cardiovascular Sciences, School of Life Science, Shanghai University, and his team screened the lncRNAs in contributing the modulation of exercise-induced cardiac growth through lncRNA microarray profiling and found that CPhar was increased with exercise and was necessary for exercise-induced physiological cardiac growth[3]. Overexpression of CPhar prevented myocardial ischemia reperfusion injury and cardiac dysfunction in vivo. They identified DDX17 (DEAD-Box Helicase 17) as a binding partner of CPhar in regulating CPhar downstream factor ATF7 (activating transcription factor 7) by sequestering C/EBPβ (CCAAT/enhancer binding protein beta) (Fig.3). The cardioprotective exercise–induced lncRNA CPhar might have translational values and may also inform new strategies and opportunities for cardiac repair.
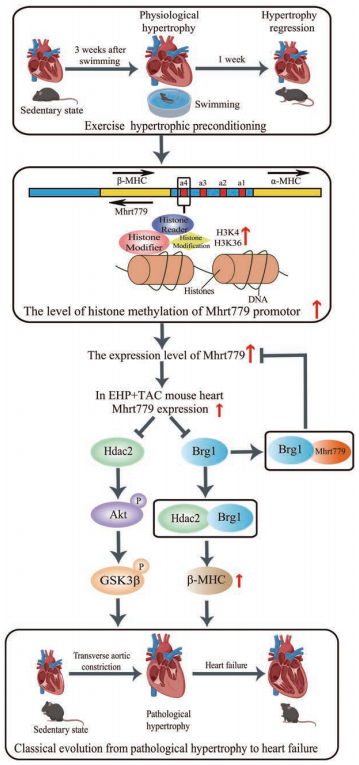
Fig.2 Exercise-induced Mhrt779 inhibits myocardial hypertrophic
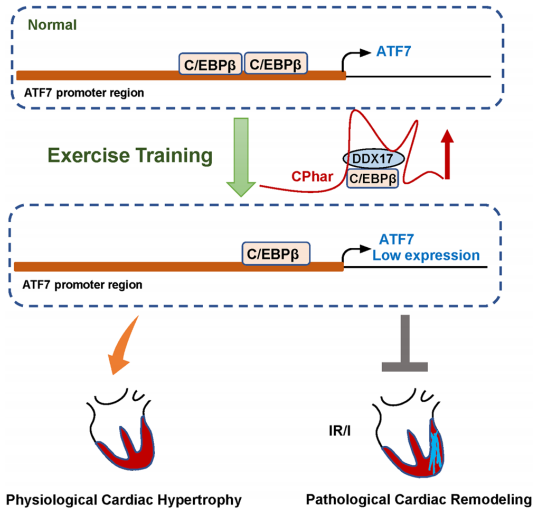
Fig.3 long noncoding RNA CPhar induces myocardial hypertrophy hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury
References
[1]Jiang X, Shao M, Liu X, et al. Reversible Treatment of Pressure Overload-Induced Left Ventricular Hypertrophy through Drd5 Nucleic Acid Delivery Mediated by Functional Polyaminoglycoside [J]. Adv Sci (Weinh). 2021 Jan 6, 8(5):2003706. (IF=16.806)
[2]Lin H, Zhu Y, Zheng C, et al. Antihypertrophic Memory After Regression of Exercise-Induced Physiological Myocardial Hypertrophy Is Mediated by the Long Noncoding RNA Mhrt779 [J]. Circulation. 2021 Jun 8, 143(23):2277-2292.(IF=29.690)
[3]Gao R, Wang L, Bei Y, et al. Long Noncoding RNA Cardiac Physiological Hypertrophy-Associated Regulator Induces Cardiac Physiological Hypertrophy and Promotes Functional Recovery After Myocardial Ischemia-Reperfusion Injury [J]. Circulation. 2021 Jul 27, 144(4):303-317. (IF=29.690)
Cloud-Clone can not only provide a variety of cardiovascular system disease models, including myocardial hypertrophy, myocardial infarction, arrhythmia, myocarditis, heart failure, etc., covering common cardiovascular system diseases; It also has various kinds of cardiovascular system signal pathway common indicators related products, which can help the majority of researchers to study cardiovascular system diseases.