Targeting gut microbiota to improve metabolic related diseases
Gut microbiota is a complex community of microorganisms that live in the digestive tracts of humans and animals, which can synthesize various metabolic products that affect human health either positively or negatively, upon interaction with the host. Gut bacteria could metabolize indigestible carbohydrates like cellulose, hemicelluloses, resistant starch, pectin, oligosaccharides and lignin into short chain fatty acids (SCFAs) such as acetic, propionic and butyric acids. The microbiota can also perform an essential role in vitamin synthesis such as biotin, thiamine, cobalamin, riboflavin, nicotine and pantothenic acids, as well as vitamin B and K. Observational findings achieved during the past two decades suggest that the intestinal microbiota may contribute to the metabolic health of the human host and, when aberrant, to the pathogenesis of various common metabolic disorders including obesity, type 2 diabetes, non-alcoholic liver disease, cardio-metabolic diseases and malnutrition. Recently, multiple studies have discussed the effects of gut microbiota and derived microbial compounds on the metabolism of healthy hosts or common metabolic diseases, which may provide assistance for the treatment of related diseases.
1. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid
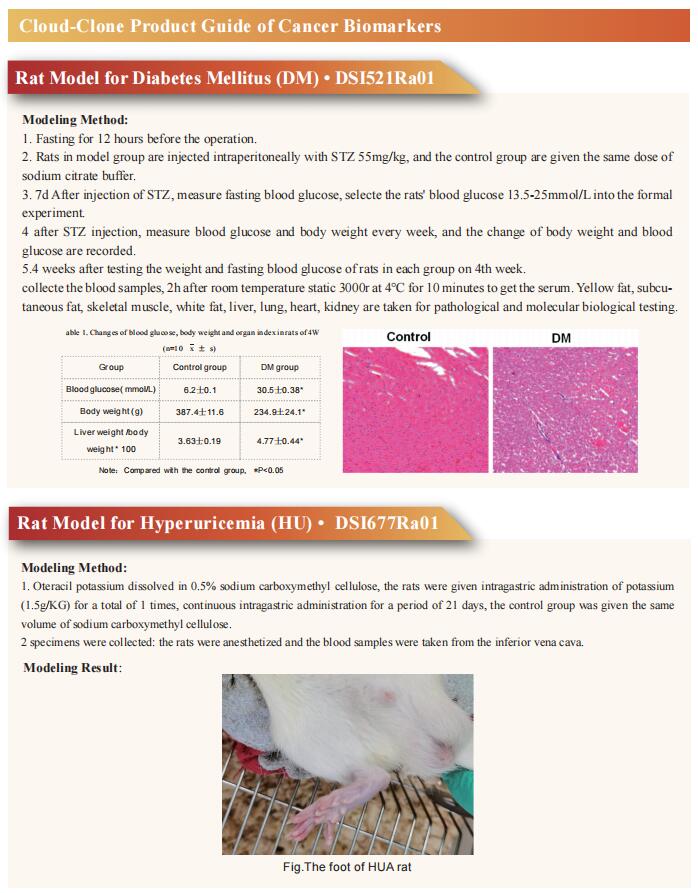
Non-alcoholic steatohepatitis (NASH) is the severe form of non-alcoholic fatty liver disease, and is characterized by liver inflammation and fat accumulation. Dietary interventions, such as fibre, have been shown to alleviate this metabolic disorder in mice via the gut microbiota. Jun Yu, Institute of Digestive Disease and Department of Medicine and Therapeutics, State Key Laboratory of Digestive Disease, Li Ka Shing Institute of Health Sciences, CUHK Shenzhen Research Institute, The Chinese University of Hong Kong, China, and his team investigated the mechanistic role of the gut microbiota in ameliorating NASH via dietary fibre in mice[1]. Soluble fibre inulin was found to be more effective than insoluble fibre cellulose to suppress NASH progression in mice, as shown by reduced hepatic steatosis, necro-inflammation, ballooning and fibrosis. They employed stable isotope probing to trace the incorporation of 13C-inulin into gut bacterial genomes and metabolites during NASH progression. Shotgun metagenome sequencing revealed that the commensal Parabacteroides distasonis was enriched by 13C-inulin. Integration of 13C-inulin metagenomes and metabolomes suggested that P. distasonis used inulin to produce pentadecanoic acid, an odd-chain fatty acid, which was confirmed in vitro and in germ-free mice. P. distasonis or pentadecanoic acid was protective against NASH in mice. Mechanistically, inulin, P. distasonis or pentadecanoic acid restored gut barrier function in NASH models (Fig.1), which reduced serum lipopolysaccharide and liver pro-inflammatory cytokine expression. Overall this shows that gut microbiota members can use dietary fibre to generate beneficial metabolites to suppress metabolic disease.
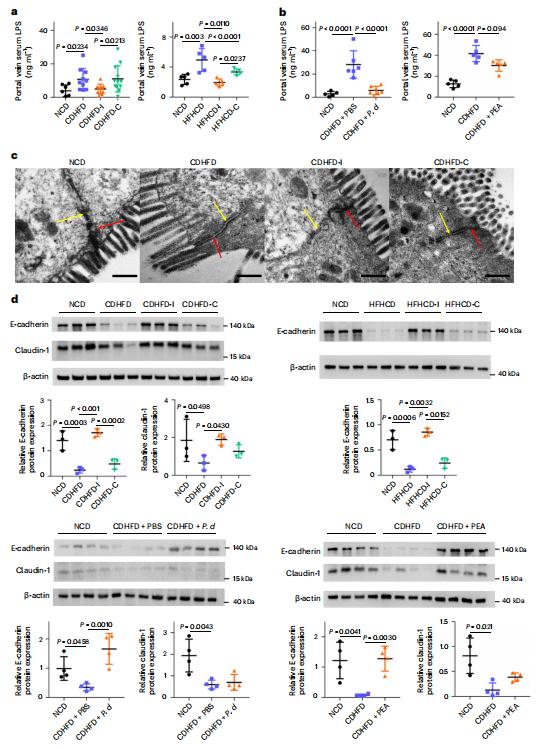
Fig.1 Inulin, P. distasonis and pentadecanoic acid restored gut barrier function
2. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9
The intestinal microbiota regulates mammalian lipid absorption, metabolism, and storage. Lora V. Hooper, Department of Immunology, The University of Texas Southwestern Medical Center, USA, and his team reported that the microbiota reprograms intestinal lipid metabolism in mice by repressing the expression of long noncoding RNA (lncRNA) Snhg9 (small nucleolar RNA host gene 9) in small intestinal epithelial cells[2]. Using RNA sequencing technology, they compared gene expression in the small intestine of germ-free mice and "conventional" mice with typical gut bacteria, and then locked in a gene called Snhg9. Snhg9 suppressed the activity of peroxisome proliferator-activated receptor g (PPARg), a central regulator of lipid metabolism, by dissociating the PPARg inhibitor sirtuin 1 from cell cycle and apoptosis protein 2 (CCAR2). Forced expression of Snhg9 in the intestinal epithelium of conventional mice impaired lipid absorption, reduced body fat, and protected against diet-induced obesity (Fig.2). The microbiota repressed Snhg9 expression through an immune relay encompassing myeloid cells and group 3 innate lymphoid cells. These results could suggest strategies for treating metabolic disease by targeting Snhg9 and the microbiota.
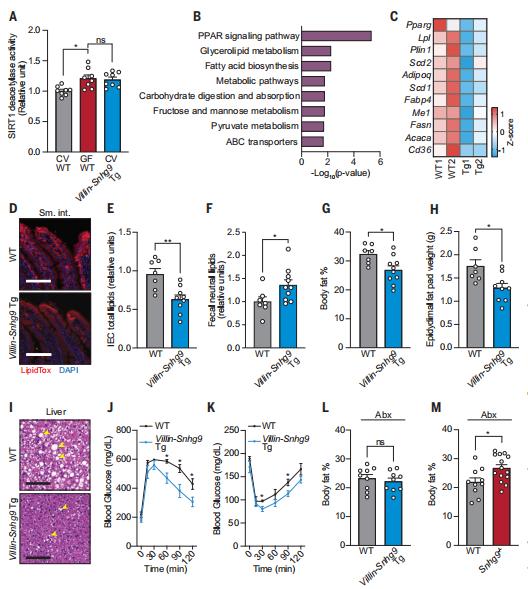
Fig.2 Villin-Snhg9 transgenic mice have reduced lipid absorption and are protected from high-fat diet-induced metabolic disorders
3. Total parenteral nutrition impairs glucose metabolism by modifying the gut microbiome
Total parenteral nutrition (TPN) can lead to complications, such as glucose metabolism disorders. While TPN is associated with impairments in intestinal function, the gut barrier and mucosal immunity, the relationship between the gut microbiome and TPN-related glucose metabolism disorders remains to be explored. In a cohort of 256 participants with type 2 intestinal failure, Xinying Wang, Department of General Surgery, Jinling Hospital, Medical School of Nanjing University, China, and his team showed that parenteral nutrition providing >80% of total energy induces insulin resistance and a higher risk of complications[3]. Using various male mouse models, they demonstrate that changes in Lactobacillaceae and indole-3-acetic acid (IAA) levels underlie these complications. Lactobacillaceae and IAA levels decrease in TPN-treated mice and participants, while their abundances in the latter are negatively correlated with insulin resistance and serum lipopolysaccharide levels. Furthermore, IAA activates the aryl hydrocarbon receptor and increases glucagon-like peptide-1 secretion through upregulation of Gcg expression and increased stem cell diferentiation towards L cells (Fig.3). Finally, liraglutide, a glucagon-like peptide-1 receptor agonist, completely prevents TPN-induced glucose metabolism disorders in mice. This study highlights that GLP-1 supplementation and manipulation of the gut microbiota and its metabolites might represent effective therapeutic strategies to prevent TPN-induced glucose metabolism disorders and their complications.
Fig.3 Inactivation of indole/AhR signalling inhibits GLP-1 production and AhR ligands can influence the ability of L cells to secrete GLP-1 through multiple pathways
References
[1]Wei W, Wong CC, Jia Z, et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat Microbiol. 2023;8(8):1534-1548. (IF=28.3)
[2]Wang Y, Wang M, Chen J, et al. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. Science. 2023;381(6660):851-857. (IF=56.9)
[3]Wang P, Sun H, Maitiabula G, et al. Total parenteral nutrition impairs glucose metabolism by modifying the gut microbiome. Nat Metab. 2023;5(2):331-348. (IF=20.8)
Cloud-Clone can provide a variety of animal models of metabolic disorders, including diabetes, hyperuricemia, hyperglycemia, nonalcoholic fatty liver, hyperlipidemia, atherosclerosis and other common diseases. We also have various metabolic disorder detection indicators and the above-mentioned PPARg, sirtuin 1, IAA, Gcg, GLP-1 and other related products, which can assist researchers in conducting research related to metabolic diseases.