The role of macrophages in cardiovascular disease
The cardiovascular system is composed of the heart and blood vessels. The heart sends blood to the arteries of the whole body, and exchanges nutrients, oxygen and metabolic waste through capillaries. After that, the blood enters the veins and is transported back to the heart by the veins of the whole body to complete the circulation. When the heart and its connected blood vessels appear pathological changes, it is called cardiovascular disease, including insufficient blood supply to the heart, arrhythmia, cardiac hypertrophy, cardiovascular disease, etc., which can affect the function of the cardiovascular system.
Macrophages
As an important component of innate immunity, macrophages are widely found in tissue structures including heart, and play an important role in development, physiological homeostasis, tissue repair and remodeling, and immune response. Cardiac macrophages are heterogeneous and have different functions in different stages of heart development or under different physiological and pathological conditions.
1. Macrophages and the adaptive growth of the heart
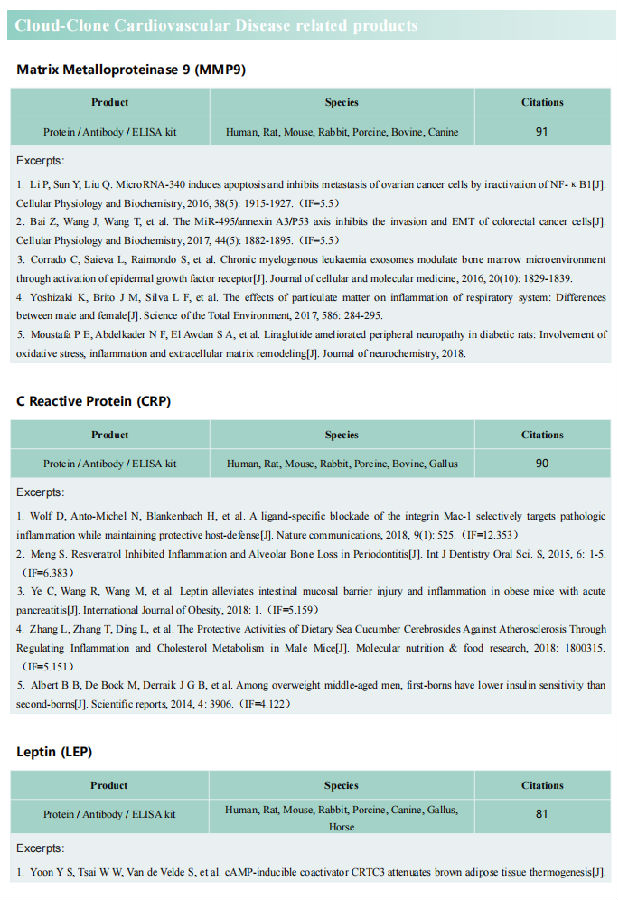
Cardiomyocyte growth and increased cardiac mass are essential to withstand hypertensive stress. In August 2021, Slava Epelman team from Toronto General Hospital Research Institute published a paper in Immunity, reported that Hypertension drove selective in situ proliferation and transcriptional activation of some cardiac resident macrophages(RM) states, directly correlating with increased cardiomyocyte growth[1]. During hypertension, inducible ablation of RMs or selective deletion of RM-derived Igf1 prevented adaptive cardiomyocyte growth, and cardiac mass failed to increase, which led to cardiac dysfunction(Fig 1). These data reveal the essential role of cardiac RM-derived IGF-1 in mediating adaptive cardiac growth during hypertension.
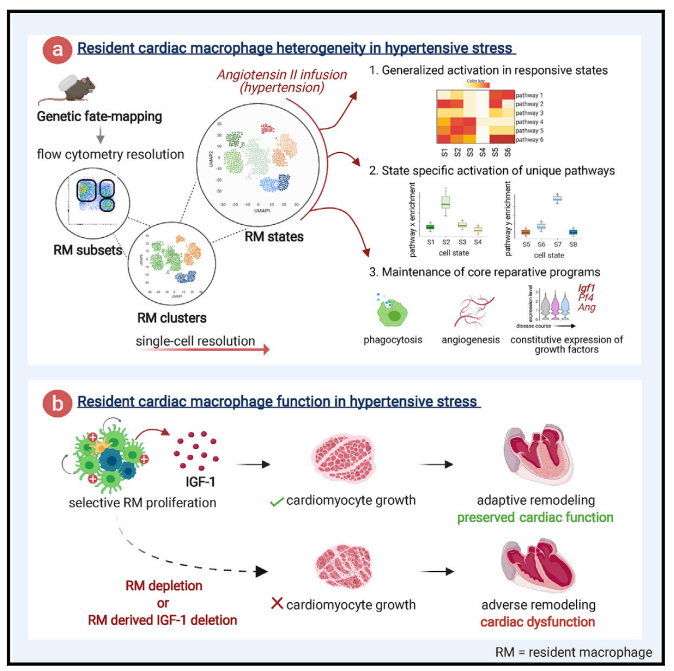
Fig 1. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress
2. Macrophages and chronic heart failure
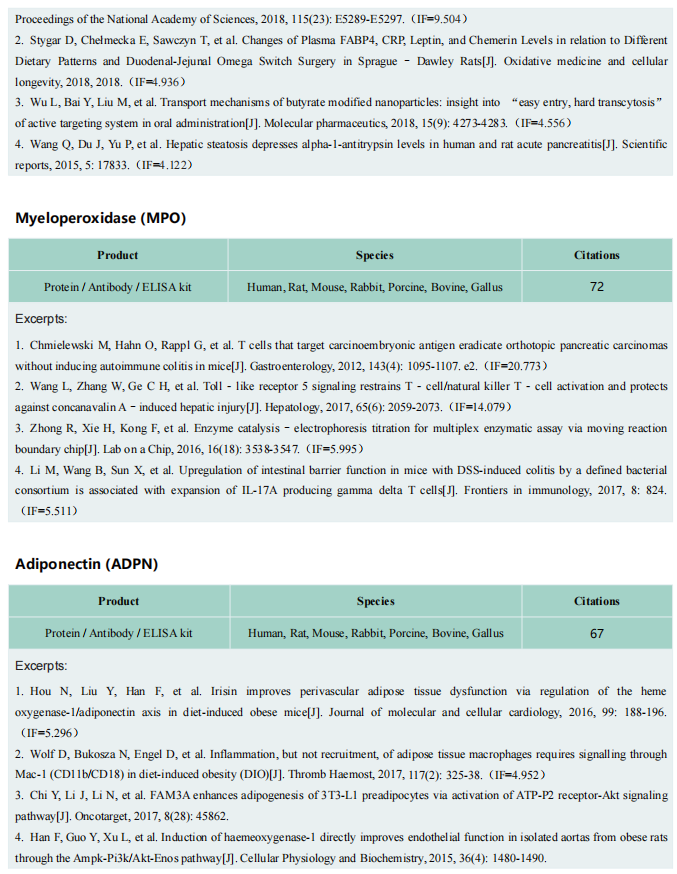
Recent studies have revealed that C-C Chemokine Receptor 2 positive (CCR2+ ) macrophages derived from infiltrating monocytes regulate myocardial inflammation and heart failure pathogenesis. Comparatively little is known about the functions of tissue resident (CCR2-) macrophages. In July 2021, Kory J. Lavine team from Washington University School of Medicine published a paper in Immunity, which identified an essential role for CCR2- macrophages in the chronically failing heart[2]. They demonstrated that CCR2- macrophages maintain adequate cardiac output in the setting of reduced cardiac contractility by promoting left ventricle enlargement and expansion of the coronary system at the macrovascular and microvascular levels. CCR2- macrophages stably interact with neighboring cardiomyocytes through focal adhesion complexes and sense mechanical stretch. Mechanical stretch serves as a stimulus for proangiogenic growth-factor expression, a process that was dependent on TRPV4(Fig 2). These findings establish a role for tissue-resident macrophages in adaptive cardiac remodeling and implicate mechanical sensing in cardiac macrophage activation.
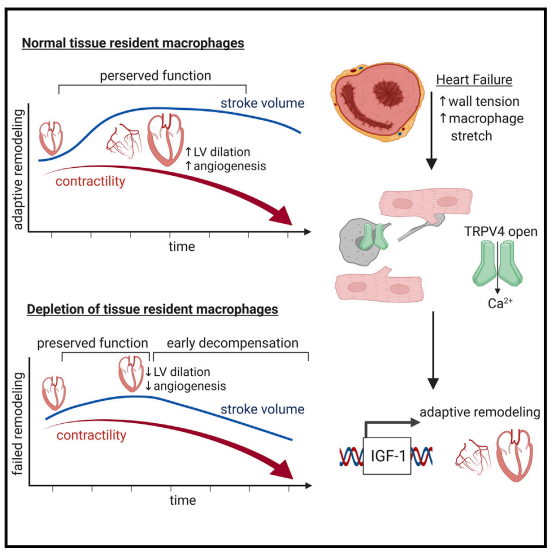
Fig 2. Resident cardiac macrophages mediate adaptive myocardial remodeling
3. Macrophages and aortic aneurysm and dissection
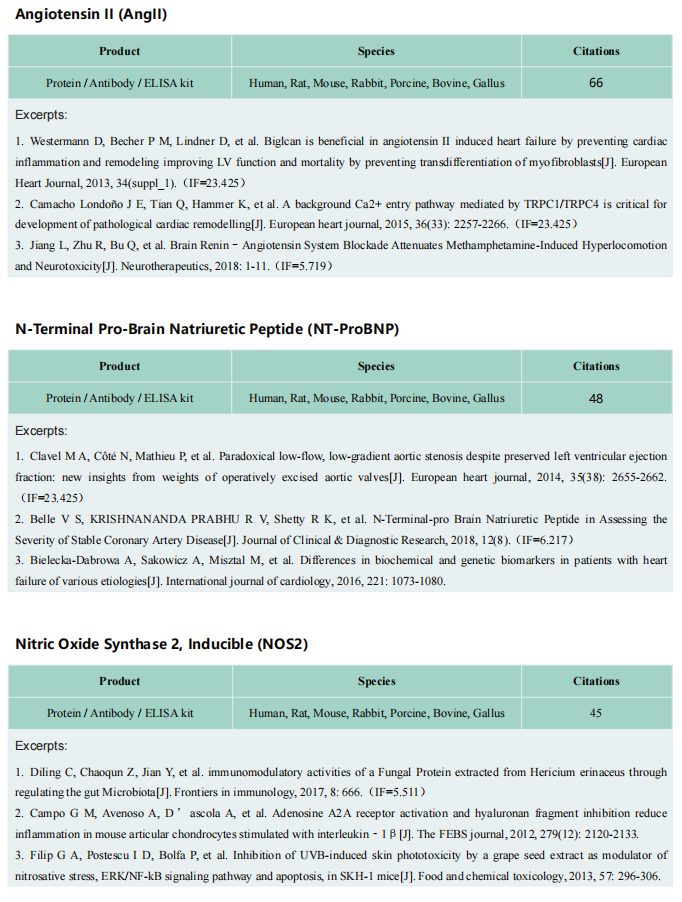
Aortic aneurysm and dissection (AAD) are high-risk cardiovascular diseases with no effective cure. On 17 September, 2021, the paper jointly published in the European Heart Journal by Lemin Zheng, Peking University, and Daowen Wang, Tongji Hospital, Huazhong University of Science and Technology revealed that succinate plays an important role in the pathogenesis of AAD and can potentially be used as a new biomarker for diagnosis[3]. Macrophages accumulate in AAD tissues and may release succinate into the blood during AAD, causing an increase in plasma succinate level, which in turn, aggravates the progression of AAD. The p38a–CREB–OGDH axis regulates the production of succinate in macrophages. Deletion of p38a could inhibit the progression of AAD. These data provide a rationale for targeting succinate-related pathways as a new target for AAD treatment.
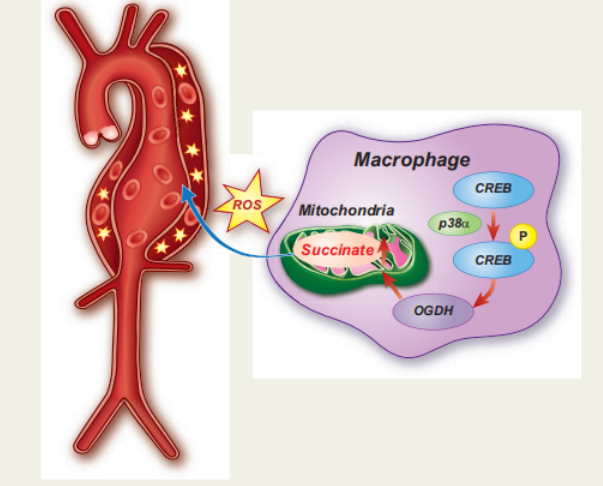
Fig 3. succinate produced by macrophages promotes the progression of aortic aneurysms and dissections
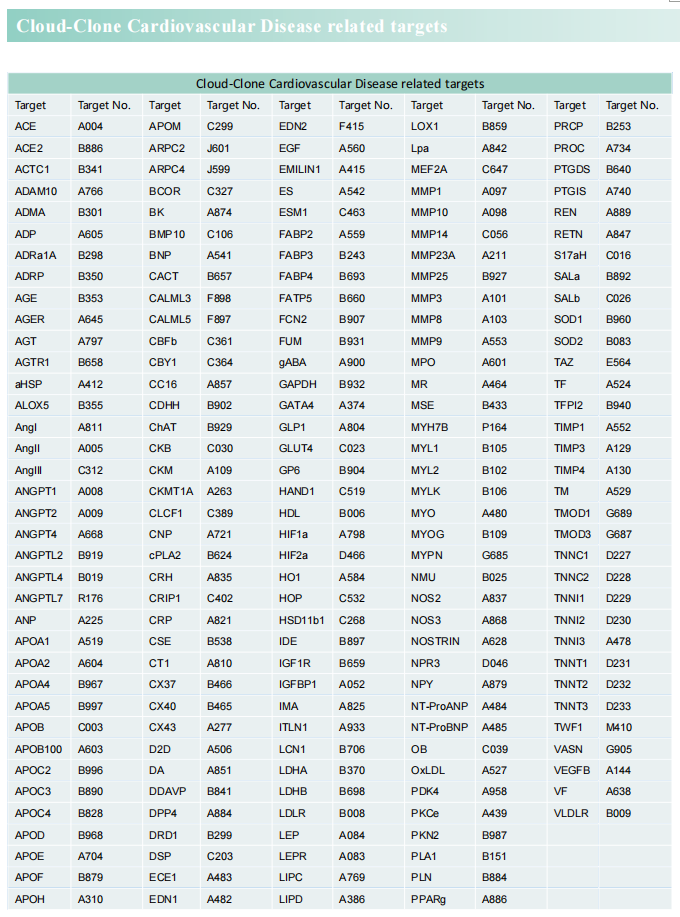
Cloud-Clone can not only provide a variety of animal models of cardiovascular system diseases, covering common diseases such as hypertension, atherosclerosis, myocardial infarction, and heart failure. It also has various indicators for the detection of cardiovascular system diseases and the proteins involved in the above-mentioned research (LGF-1, TRPV, CCR2, CREB, OGDH), which can help the majority of scientific researchers to carry out cardiovascular system diseases related research.
Reference
[1] Zaman R, Hamidzada H, Kantores C, et al. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress[J]. Immunity, 2021, 54(9): 2057-2071.(IF=31.745)
[2] Wong NR, Mohan J, Kopecky BJ, et al. Resident cardiac macrophages mediate adaptive myocardial remodeling[J]. Immunity, 2021, 54(9): 2072-2088.(IF=31.745)
[3] Cui H, Chen Y, Li K, et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection.[J] .Eur Heart J, 2021: ehab605.(IF=29.983)
Cloud-Clone related animal models
Rat Model for Vascular Calcification (VC) • DSI845Ra01
Modeling Method
Daily vitamin D3 300,000 IU/kg body weight intramuscular injection for 4 weeks.
Nicotine gavage twice a day: Dissolve nicotine in peanut oil and intragastric administration at a dose of 25mg/kg, repeated again after 9 hours for four weeks.Note: Vitamin D3 mainly causes vascular calcification, nicotine stimulates the heartbeat, accelerates the blood pressure, and increases the blood pressure. Animal state should be observed after every intragastric administration. Mice will twitch after intragastric administration, which needs to be alleviated by manual chest compression
Mouse Model for Myocardial Infarction (MI) • DSI504Mu01
Modeling Method
The anesthetized mice were intubated and MI surgery was performed if no response was found. The mouse was decubitus on the right side. The thoracic cavity was opened to fully expose the heart, and a little pericardium was torn under the left auricle to fully expose the anterior descending branch of the left coronary artery (LAD) or its region. The coronary arteries are lapped, the chest cavity is closed, and layers of muscle and skin are sutured from the inside out.