Packages (Simulation)

Reagent Preparation
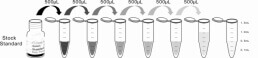
Image (I)
Image (II)
Certificate


ELISA Kit for Prostaglandin F2 Alpha (PGF2a)
PGF2A; PGF2-A; PG-F2a
- Product No.CEA749Ge
- Organism SpeciesPan-species (General) Same name, Different species.
- Sample Typeserum, plasma and other biological fluids
- Test MethodCompetitive Inhibition
- Assay Length2h
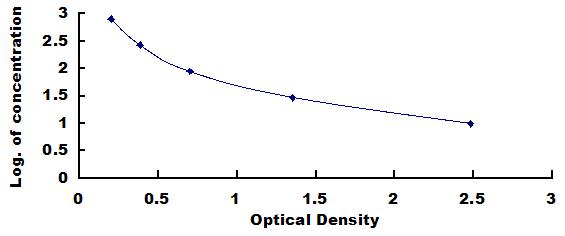
- Detection Range9.88-800pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 4.13pg/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 557
US$ 796
US$ 3580
US$ 6763
US$ 55692
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Prostaglandin F2 Alpha (PGF2a).
No significant cross-reactivity or interference between Prostaglandin F2 Alpha (PGF2a) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of Prostaglandin F2 Alpha (PGF2a) and the recovery rates were calculated by comparing the measured value to the expected amount of Prostaglandin F2 Alpha (PGF2a) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 98-105 | 101 |
| EDTA plasma(n=5) | 86-102 | 99 |
| heparin plasma(n=5) | 80-101 | 84 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Prostaglandin F2 Alpha (PGF2a) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Prostaglandin F2 Alpha (PGF2a) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Prostaglandin F2 Alpha (PGF2a) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 78-92% | 84-98% | 86-104% | 88-102% |
| EDTA plasma(n=5) | 85-94% | 91-103% | 94-101% | 85-92% |
| heparin plasma(n=5) | 87-98% | 98-105% | 78-92% | 80-92% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 50µL standard or sample to each well.
And then add 50µL prepared Detection Reagent A immediately.
Shake and mix. Incubate 1 hour at 37°C;
3. Aspirate and wash 3 times;
4. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
5. Aspirate and wash 5 times;
6. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
7. Add 50µL Stop Solution. Read at 450 nm immediately.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Pharmacognosy Reaserach | Vitex negundo?inhibits cyclooxygenase-2 inflammatory cytokine-mediated inflammation on carrageenan-induced rat hind paw edema PubMed: PMC3424839 |
| J Breath Res | Effects of different ventilation strategies on exhaled nitric oxide in geriatric abdominal surgery PubMed: 25719610 |
| Gynecol Endocrinol | Application of atosiban in frozen-thawed cycle patients with different times of embryo transfers Pubmed:27147474 |
| Fertility and Sterility | Administration of atosiban in patients with endometriosis undergoing frozen–thawed embryo transfer: a prospective, randomized study pubmed:27143518 |
| Biochemical and Biophysical Research Communications | The anti-inflammatory and anti-oxidative effects of conbercept in treatment of macular edema secondary to retinal vein occlusion Pubmed: 30558792 |
| Diabetes & Metabolic Syndrome: Clinical Research & Reviews | Association of fasting glucagon-like peptide-1 with oxidative stress and subclinical atherosclerosis in type 2 diabetes |
| Journal of Diabetes Research | Supplementation with Korean Red Ginseng Improves Current Perception Threshold in Korean Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo … Pubmed: 32025522 |
| LIFE SCIENCES | Intravitreal conbercept improves outcome of proliferative diabetic retinopathy through inhibiting inflammation and oxidative stress Pubmed: 33227274 |
| JOURNAL OF CELLULAR AND MOLECULAR MEDICINE | The microvesicle/CD36 complex triggers a prothrombotic phenotype in patients with non‐valvular atrial fibrillation Pubmed: 32510852 |
| archives of gerontology and geriatrics | The effect of Korean Red Ginseng on sarcopenia biomarkers in type 2 diabetes patients Pubmed: 32470863 |
| Catalog No. | Related products for research use of Pan-species (General) Organism species | Applications (RESEARCH USE ONLY!) |
| PAA749Ge01 | Polyclonal Antibody to Prostaglandin F2 Alpha (PGF2a) | WB; IHC; ICC; IP. |
| MAA749Ge22 | Monoclonal Antibody to Prostaglandin F2 Alpha (PGF2a) | ELISA |
| MAA749Ge23 | Monoclonal Antibody to Prostaglandin F2 Alpha (PGF2a) | ELISA |
| MAA749Ge24 | Monoclonal Antibody to Prostaglandin F2 Alpha (PGF2a) | ELISA |
| MAA749Ge21 | Monoclonal Antibody to Prostaglandin F2 Alpha (PGF2a) | ELISA |
| CEA749Ge | ELISA Kit for Prostaglandin F2 Alpha (PGF2a) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMA749Ge | Multiplex Assay Kit for Prostaglandin F2 Alpha (PGF2a) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |