Packages (Simulation)

Reagent Preparation
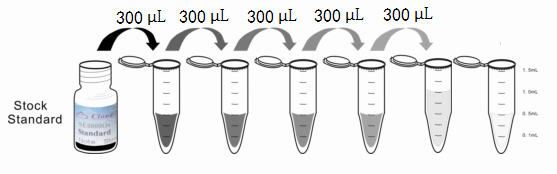
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay)
K-Glypican; Glypican Proteoglycan 4; Secreted glypican-4
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA998Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
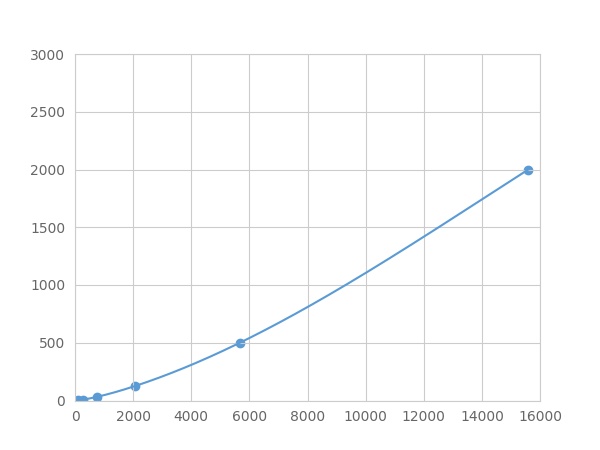
- Detection Range1.95-2000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.65 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 393
US$ 408
US$ 431
US$ 461
US$ 491
US$ 537
US$ 605
US$ 756
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 82-96 | 91 |
| EDTA plasma(n=5) | 89-96 | 92 |
| heparin plasma(n=5) | 89-101 | 93 |
| sodium citrate plasma(n=5) | 85-95 | 89 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 86-99% | 94-102% | 91-105% | 80-90% |
| EDTA plasma(n=5) | 97-105% | 79-90% | 86-93% | 83-98% |
| heparin plasma(n=5) | 91-99% | 87-104% | 78-99% | 86-101% |
| sodium citrate plasma(n=5) | 83-92% | 90-99% | 93-105% | 85-99% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:GPC4) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| PLoS One. | Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation Pubmed:Pmc4072672 |
| Aspects of fluid therapy in the critically ill. Experimental and clinical studies on fluid therapy in the inflammatory conditions. 250690_2_G5_Svajunas_S.pdf | |
| Intensive Care Medicine Experimental | Elevated plasma glypicans are associated with organ failure in patients with infection Pubmed: 30618011 |
| Crit Care | Albumin infusion rate and plasma volume expansion: a randomized clinical trial in postoperative patients after major surgery Pubmed: 31138247 |
| J Endocrinol Invest | Serum glypican4 and glycosylphosphatidylinositol-specific phospholipase D levels are associated with adipose tissue insulin resistance in obese subjects with … Pubmed: 32816247 |
| Heparin Binding Protein and Endothelial Glycocalyx Markers in Severe COVID-19–A Prospective Observational Cohort Study | |
| Sci Rep | Glypican-4 in pregnancy and its relation to glucose metabolism, insulin resistance and gestational diabetes mellitus status 34903856 |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| RPA998Hu01 | Recombinant Glypican 4 (GPC4) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAA998Hu01 | Polyclonal Antibody to Glypican 4 (GPC4) | WB; IHC; ICC; IP. |
| LAA998Hu81 | FITC-Linked Polyclonal Antibody to Glypican 4 (GPC4) | WB; IHC; ICC; IF. |
| LAA998Hu71 | Biotin-Linked Polyclonal Antibody to Glypican 4 (GPC4) | WB; IHC; ICC. |
| MAA998Hu22 | Monoclonal Antibody to Glypican 4 (GPC4) | WB; IHC; ICC; IP. |
| SEA998Hu | ELISA Kit for Glypican 4 (GPC4) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMA998Hu | Multiplex Assay Kit for Glypican 4 (GPC4) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |