Packages (Simulation)

Reagent Preparation
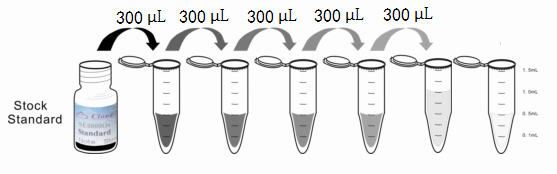
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay)
CF-B; BF; BFD; CFAB; GBG; H2-Bf; PBF2; B-Factor; Properdin; Properdin factor B; C3/C5 convertase; Glycine-rich beta glycoprotein
(Note: Up to 8-plex in one testing reaction)
- Product No.LMC011Si
- Organism SpeciesRhesus monkey (Simian) Same name, Different species.
- Sample TypeSerum, plasma and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
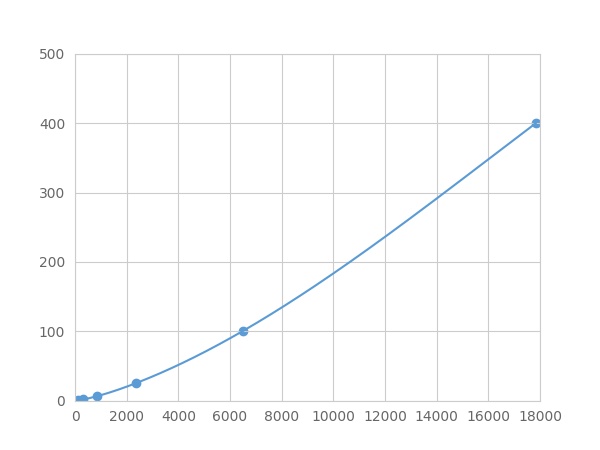
- Detection Range0.39-400ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.13 ng/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 562
US$ 583
US$ 616
US$ 659
US$ 702
US$ 767
US$ 864
US$ 1080
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 94-101 | 98 |
| EDTA plasma(n=5) | 80-101 | 82 |
| heparin plasma(n=5) | 79-99 | 91 |
| sodium citrate plasma(n=5) | 93-102 | 97 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 79-96% | 95-105% | 93-105% | 92-101% |
| EDTA plasma(n=5) | 85-101% | 85-101% | 82-94% | 78-93% |
| heparin plasma(n=5) | 81-101% | 80-92% | 78-89% | 85-93% |
| sodium citrate plasma(n=5) | 92-99% | 85-99% | 79-102% | 95-103% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:CFB) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| BMC Nephrology | Complement in patients receiving maintenance hemodialysis: functional screening and quantitative analysis BioMed: 14712369 |
| J. Proteome Res. | Identification and Confirmation of Differentially Expressed Fucosylated Glycoproteins in the Serum of Ovarian Cancer Patients Using a Lectin Array and LC–MS/MS ACS: pr300330z |
| Pediatr Int | Atypical hemolytic uremic syndrome: Korean pediatric series PubMed: 25443527 |
| Journal of Neuroimmunology | Acute and prolonged complement activation in the central nervous system during herpes simplex encephalitis Pubmed:27235358 |
| Biochemical and Biophysical Research Communications | Adipose tissue complement factor B promotes adipocyte maturation. pubmed:29137982 |
| Journal of proteome research | Serum exosomes from newborn piglets restrict porcine epidemic diarrhea virus infection |
| journal of human genetics | Identification of susceptibility locus shared by IgA nephropathy and inflammatory bowel disease in a Chinese Han population Pubmed: 31857673 |
| Nephrol Dial Transplant | Association between thrombotic microangiopathy and activated alternative complement pathway in malignant nephrosclerosis 33367879 |
| JOURNAL OF GENERAL VIROLOGY | Changes in complement alternative pathway components, factor B and factor H during dengue virus infection in the AG129 mouse 33410734 |
| J Proteome Res | Early Diagnostic Ability of Human Complement Factor B in Pancreatic Cancer Is Partly Linked to Its Potential Tumor-Promoting Role 34766501 |
| J Clin Med | A Three-Protein Panel to Support the Diagnosis of Sepsis in Children Pubmed:35329889 |
| Catalog No. | Related products for research use of Rhesus monkey (Simian) Organism species | Applications (RESEARCH USE ONLY!) |
| RPC011Si01 | Recombinant Complement Factor B (CFB) | Positive Control; Immunogen; SDS-PAGE; WB. |
| SEC011Si | ELISA Kit for Complement Factor B (CFB) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMC011Si | Multiplex Assay Kit for Complement Factor B (CFB) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |