Packages (Simulation)

Reagent Preparation
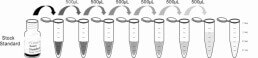
Image (I)
Image (II)
Certificate


ELISA Kit for Erythropoietin (EPO)
EP; Epoetin; Erythropoetin; Hematopoietin; Hemopoietin
- Product No.SEA028Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3h
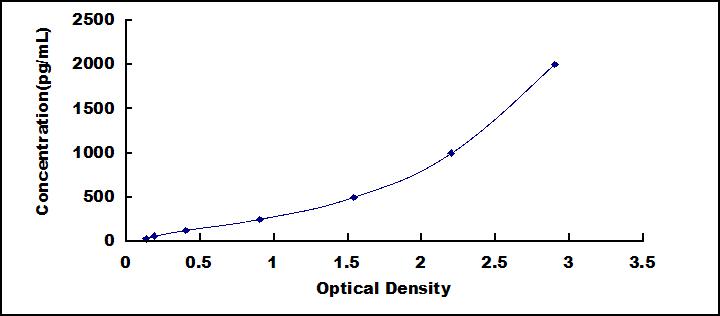
- Detection Range31.2-2,000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 11.7pg/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 412
US$ 588
US$ 2646
US$ 4998
US$ 41160
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Erythropoietin (EPO).
No significant cross-reactivity or interference between Erythropoietin (EPO) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Erythropoietin (EPO) and the recovery rates were calculated by comparing the measured value to the expected amount of Erythropoietin (EPO) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 95-104 | 101 |
| EDTA plasma(n=5) | 86-95 | 91 |
| heparin plasma(n=5) | 78-98 | 86 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Erythropoietin (EPO) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Erythropoietin (EPO) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Erythropoietin (EPO) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 87-94% | 91-101% | 84-98% | 81-101% |
| EDTA plasma(n=5) | 80-89% | 93-101% | 80-97% | 85-93% |
| heparin plasma(n=5) | 78-103% | 88-95% | 83-91% | 91-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
GIVEAWAYS
INCREMENT SERVICES
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
| Magazine | Citations |
| Endocrinology | Prenatal Glucocorticoid Overexposure Causes Permanent Increases in Renal Erythropoietin Expression and Red Blood Cell Mass in the Rat Offspring Endo: source |
| Journal of Ethnopharmacology | Inhibitory effect of polysaccharides isolated from Angelica sinensis on hepcidin expression PubMed: 21333724 |
| PLOS ONE | CD98 Positive Eosinophils Contribute to T Helper 1 Pattern Inflammation PlosOne: Source |
| The Journal of Clinical Endocrinology & Metabolism | Alterations of Circulating Endothelial Cell and Endothelial Progenitor Cell Counts around the Ovulation PubMed: 22948762 |
| Experimental & Molecular Medicine | Long-term and stable correction of uremic anemia by intramuscular injection of plasmids containing hypoxia-regulated system of erythropoietin expression PubMed: PMC3509184 |
| Biochem Biophys Res Commun. | Reevaluation of erythropoietin production by the nephron Pubmed:24832733 |
| Nutrition. | Carbohydrate and glutamine supplementation modulates the Th1/Th2 balance after exercise performed at a simulated altitude of 4500 m. Pubmed:2528040 |
| J Appl Physiol (1985). | Decreased plasma soluble erythropoietin receptor in high-altitude excessive erythrocytosis and Chronic Mountain Sickness Pubmed:25324511 |
| Expert Rev Proteomics | Plasma hepcidin in early-stage breast cancer patients: no relationship with interleukin-6, erythropoietin and erythroferrone PubMed: 26496240 |
| Oncotarget | KIAA0101 is associated with human renal cell carcinoma proliferation and migration induced by erythropoietin pubmed:26575329 |
| international journal of molecular sciences | Expression of Iron-Related Proteins Differentiate Non-Cancerous and Cancerous Breast Tumors. pubmed:28216608 |
| Veterinary Clinical Pathology | Occult gastrointestinal bleeding is a common finding in dogs with chronic kidney disease pubmed:28186645 |
| IOS Press Content Library | An Intranasal Formulation of Erythropoietin (Neuro-EPO) Prevents Memory Deficits and Amyloid Toxicity in the APPSwe Transgenic Mouse Model of Alzheimer's … articles:journal-of-alzheimers-disease |
| Oxidative Medicine and Cellular Longevity | Acidic Polysaccharide from Angelica sinensis Reverses Anemia of Chronic Disease Involving the Suppression of Inflammatory Hepcidin and NF-κB Activation pubmed:29147463 |
| PLoS Neglected Tropical Diseases | Functional and phenotypic evaluation of eosinophils from patients with the acute form of paracoccidioidomycosis pubmed:28489854 |
| Neurochemistry International | Effects of erythropoietin on astrocytes and brain endothelial cells in primary culture during anoxia depend on simultaneous signaling by other cytokines and on duration of anoxia S0197018617304783 |
| Pesquisa Veterinária Brasileira | Relation between anaemia and bone marrow features and serum erythropoietin in dogs with chronic kidney disease 10.1590/s0100-736x2017000600011 |
| Medical Journal Armed Forces India | Study of acute hypoxia markers in healthy subjects: Utility in post-crash investigation 10.1016/j.mjafi.2017.04.003 |
| Neurochemistry International | Effects of erythropoietin on astrocytes and brain endothelial cells in primary culture during anoxia depend on simultaneous signaling by other cytokines and on … Pubmed:29180303 |
| Clinical and Experimental Medicine | Increased NGAL level associated with iron store in chronic kidney disease with anemia Pubmed:29909502 |
| Bulletin of Experimental Biology and Medicine | Signal Mechanism of the Protective Effect of Combined Preconditioning by Amtizole and Moderate Hypoxia Pubmed:29308565 |
| Bulletin of Experimental Biology and Medicine | Expression of Hif-1α, Nf-κb, and Vegf Genes in the Liver and Blood Serum Levels of HIF-1α, Erythropoietin, VEGF, TGF-β, 8-Isoprostane, and Corticosterone in Wistar Rats with High and Low Resistance to Hypoxia. Corticosterone in … Pubmed: 30353332 |
| FASEB JOURNAL | High-altitude hypoxia decreases plasma erythropoietin soluble receptor (sEpoR) concentration in humans |
| Journal of Functional Foods | Dietary gelatin enhances non-heme iron absorption possibly via regulation of systemic iron homeostasis in rats |
| 博士论文 | PHYSIOLOGICAL ADAPTATIONS TO HEAT ACCLIMATION; REPERCUSSIONS ON CYCLING PERFORMANCE |
| Nutrition | Effects of carbohydrate and glutamine supplementation on cytokine production by monocytes after exercise in hypoxia: a crossover, randomized and double-blind … Pubmed: 31743809 |
| BRITISH JOURNAL OF HAEMATOLOGY | Disordered serum erythroferrone and hepcidin levels as indicators of the spontaneous abortion occurrence during early pregnancy in humans Pubmed: 32866306 |
| JCI Insight. | Increased FGF-23 levels are linked to ineffective erythropoiesis and impaired bone mineralization in myelodysplastic syndromes Pubmed: 32759495 |
| Blood Cells, Molecules, and Diseases | EPAS1 regulates proliferation of erythroblasts in chronic mountain sickness Pubmed: 32470757 |
| Int J Endocrinol | miR663 Prevents Epo Inhibition Caused by TNF-Alpha in Normoxia and Hypoxia 34367277 |
| J Mater Sci Mater Med | Loading of erythropoietin on biphasic calcium phosphate bioceramics promotes osteogenesis and angiogenesis by regulating EphB4/EphrinB2 molecules Pubmed:35072831 |
| Effect of arsenate on erythropoietin production and autophagy induction in HepG2 cells |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| APA028Hu61 | Active Erythropoietin (EPO) | Cell culture; Activity Assays. |
| EPA028Hu61 | Eukaryotic Erythropoietin (EPO) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPA028Hu01 | Recombinant Erythropoietin (EPO) | Positive Control; Immunogen; SDS-PAGE; WB. |
| APA028Hu01 | Active Erythropoietin (EPO) | Cell culture; Activity Assays. |
| APA028Hu62 | Active Erythropoietin (EPO) | Cell culture; Activity Assays. |
| PAA028Hu06 | Polyclonal Antibody to Erythropoietin (EPO) | WB; IHC |
| PAA028Hu01 | Polyclonal Antibody to Erythropoietin (EPO) | IHC |
| LAA028Hu71 | Biotin-Linked Polyclonal Antibody to Erythropoietin (EPO) | WB; IHC; ICC. |
| MAA028Hu23 | Monoclonal Antibody to Erythropoietin (EPO) | WB |
| MAA028Hu26 | Monoclonal Antibody to Erythropoietin (EPO) | WB |
| MAA028Hu21 | Monoclonal Antibody to Erythropoietin (EPO) | IHC |
| MAA028Hu24 | Monoclonal Antibody to Erythropoietin (EPO) | IHC |
| MAA028Hu25 | Monoclonal Antibody to Erythropoietin (EPO) | IHC |
| MAA028Hu22 | Monoclonal Antibody to Erythropoietin (EPO) | WB; IHC |
| SEA028Hu | ELISA Kit for Erythropoietin (EPO) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SCA028Hu | CLIA Kit for Erythropoietin (EPO) | Chemiluminescent immunoassay for Antigen Detection. |
| LMA028Hu | Multiplex Assay Kit for Erythropoietin (EPO) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |
| KSA028Hu01 | ELISA Kit DIY Materials for Erythropoietin (EPO) | Main materials for "Do It (ELISA Kit) Yourself". |