Packages (Simulation)

Reagent Preparation
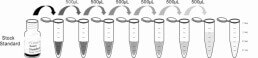
Image (I)
Image (II)
Certificate


- Featured-Product
ELISA Kit for Retinol Binding Protein 4 (RBP4)
PRBP; RBP; Plasma retinol-binding protein; Retinol Binding Protein 4, Plasma
- Product No.SEA929Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3h
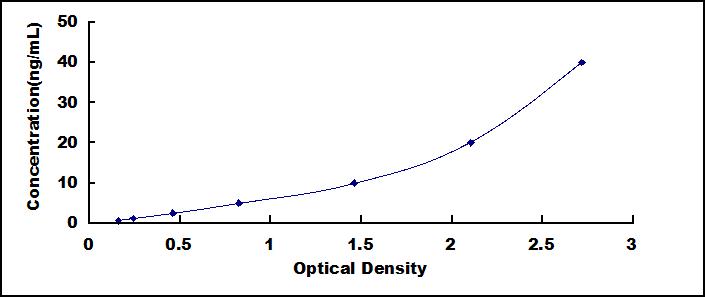
- Detection Range0.625-40ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.284ng/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 349
US$ 498
US$ 2241
US$ 4233
US$ 34860
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Retinol Binding Protein 4 (RBP4).
No significant cross-reactivity or interference between Retinol Binding Protein 4 (RBP4) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Retinol Binding Protein 4 (RBP4) and the recovery rates were calculated by comparing the measured value to the expected amount of Retinol Binding Protein 4 (RBP4) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 96-103 | 99 |
| EDTA plasma(n=5) | 84-99 | 90 |
| heparin plasma(n=5) | 92-101 | 97 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Retinol Binding Protein 4 (RBP4) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Retinol Binding Protein 4 (RBP4) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Retinol Binding Protein 4 (RBP4) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 92-101% | 83-104% | 96-104% | 96-105% |
| EDTA plasma(n=5) | 80-102% | 90-98% | 81-97% | 87-94% |
| heparin plasma(n=5) | 84-92% | 95-105% | 78-91% | 92-99% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
GIVEAWAYS
INCREMENT SERVICES
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
| Magazine | Citations |
| Diabetes Technology & Therapeutics | Proteomic Identification of Human Urinary Biomarkers in Diabetes Mellitus Type 2 Liebert: 20100078 |
| Journal of Pharmaceutical and Biomedical Analysis | Effect of high dose thiamine on the levels of urinary protein biomarkers in diabetes mellitus type 2 PubMed: 21130593 |
| Evid Based Complement Alternat Med | Quercetin Protects against Cadmium-Induced Renal Uric Acid Transport System Alteration and Lipid Metabolism Disorder in Rats PubMed: PMC3368504 |
| Journal of Endocrinology | Combined galanin with insulin improves insulin sensitivity of diabetic rat muscles Pubmed:24501381 |
| J Endocrinol | Central alarin ameliorated insulin resistance of adipocytes in type 2 diabetic rats Pubmed:25240061 |
| Peptides | Effect of endogenous galanin on glucose transporter 4 expression in cardiac muscle of type 2 diabetic rats Pubmed:25445608 |
| Obesity Surgery | Long-term Effect of Ileal Transposition on Adipokine Serum Level in Zucker (Orl)-Lepr fa Fatty Rats Pubmed:25697126 |
| Clin Exp Nephrol | Differentially expressed urinary biomarkers in children with idiopathic nephrotic syndrome PubMed: 26351173 |
| Experimental and Therapeutic Medicine | Expression of retinol binding protein 4 and nuclear factor-ÎşB in diabetic rats with atherosclerosis and the intervention effect of pioglitazone Pubmed:27446311 |
| Experimental and Therapeutic Medicine | Expression of retinol binding protein 4 and nuclear factor-κB in diabetic rats with atherosclerosisand the intervention effect of pioglitazone. pubmed:27446311 |
| Journal of Chromatography A | Application of a new procedure for liquid chromatography/mass spectrometry profiling of plasma amino acid-related metabolites and untargeted shotgun proteomics to identify mechanisms and biomarkers of calcific aortic stenosis S0021967317311858 |
| BMC Cancer | Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis Pubmed:29386009 |
| Nutrition & Diabetes | Lack of pronounced changes in the expression of fatty acid handling proteins in adipose tissue and plasma of morbidly obese humans Pubmed:29335416 |
| Molecular Medicine Reports | RBP4 regulates trophoblastic cell proliferation and invasion via the PI3K/AKT signaling pathway Pubmed:30015949 |
| Relationship between retinol and risk of diabetic retinopathy: a case-control study | |
| Scientific Reports | Retinol binding protein 4 abundance in plasma and tissues is related to body fat deposition in cattle Pubmed: 31147589 |
| Potassium supplementation blunts the effects of high salt intake on serum retinol‐binding protein 4 levels in healthy individuals | |
| Docosahexaenoic acid (DHA), a nutritional supplement, modulates steroid insensitivity in asthma | |
| Eur J Nutr | The dietary inflammatory index is associated with anti-and pro-inflammatory adipokines in Brazilian schoolchildren 33575861 |
| Eur J Pediatr | Pro-and anti-inflammatory adipokines are associated with cardiometabolic risk markers in Brazilian schoolchildren 33834274 |
| Sci Rep | Ileal transposition helps to regulate plasma hepatokine levels in obese Zucker (Crl: ZUC (ORL)-Lepr fa) rats 33833309 |
| Int Arch Occup Environ Health | Biomarkers of cadmium exposure and renal function in estuarine adult villagers 34773507 |