Packages (Simulation)

Reagent Preparation
Image (I)
Image (II)
Certificate


ELISA Kit for Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13)
CD256; APRIL; TALL2; TRDL1; ZTNF2; A Proliferation Inducing Ligand; TNF And APOL Related Leukocyte Expressed Ligand 2; TNF-Related Death Ligand 1
- Product No.SEB750Mu
- Organism SpeciesMus musculus (Mouse) Same name, Different species.
- Sample Typeserum, plasma and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3h
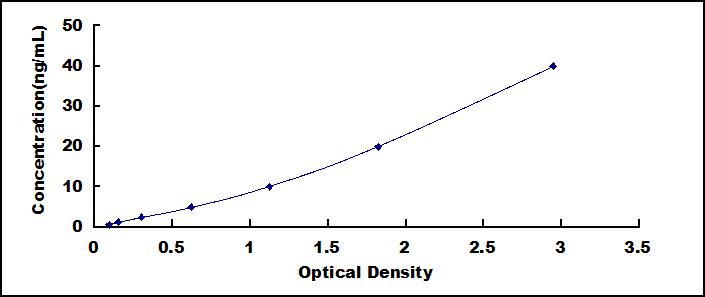
- Detection Range0.625-40ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.277ng/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 479
US$ 684
US$ 3078
US$ 5814
US$ 47880
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13).
No significant cross-reactivity or interference between Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) and the recovery rates were calculated by comparing the measured value to the expected amount of Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 91-101 | 96 |
| EDTA plasma(n=5) | 98-105 | 101 |
| heparin plasma(n=5) | 80-105 | 80 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Tumor Necrosis Factor Ligand Superfamily, Member 13 (TNFSF13) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 98-105% | 78-96% | 89-102% | 89-97% |
| EDTA plasma(n=5) | 96-105% | 89-101% | 86-94% | 96-103% |
| heparin plasma(n=5) | 95-104% | 82-98% | 83-97% | 82-102% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
GIVEAWAYS
INCREMENT SERVICES
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
| Magazine | Citations |
| Journal of Critical Care | Serum concentrations of A Proliferation-Inducing Ligand (APRIL) are elevated in sepsis and predict mortality in critically ill patients PubMed: 23337484 |
| Haematologica | Arrayed molecular barcoding identifies TNFSF13 as a positive regulator of acute myeloid leukemia-initiating cells Pubmed: 30819903 |
| Gene | A Significant Decrease of BAFF, APRIL, and BAFF Receptors Following Mesenchymal Stem Cell Transplantation in Patients with Refractory Rheumatoid Arthritis Pubmed: 31935514 |